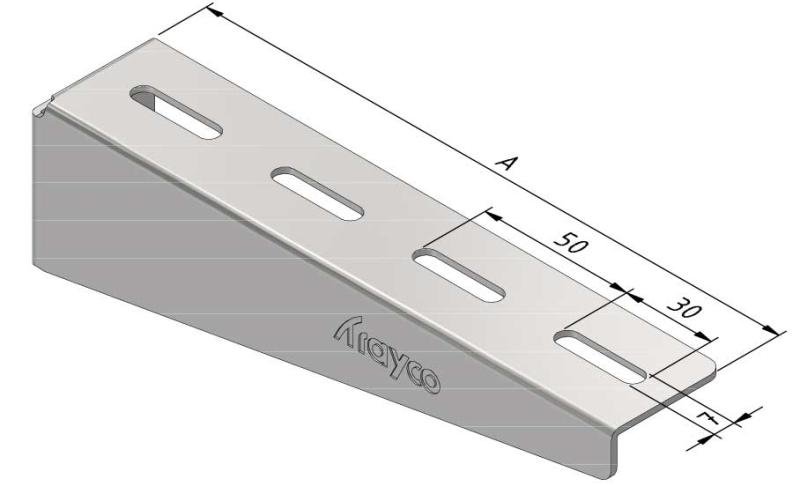
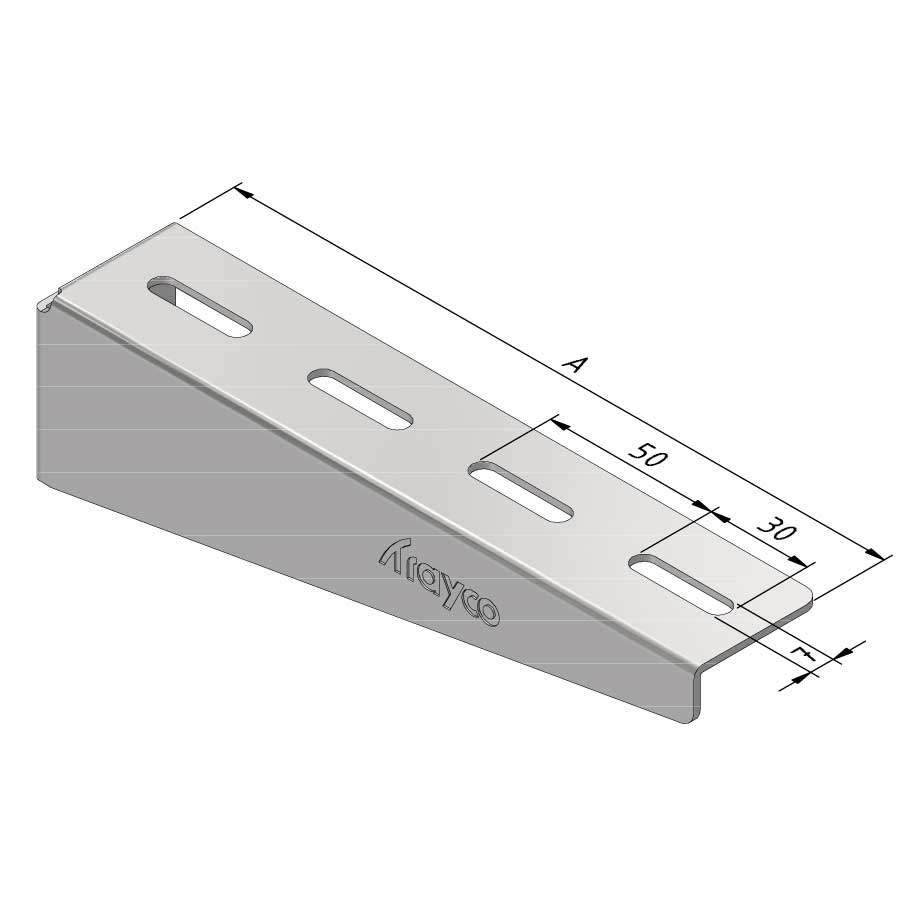
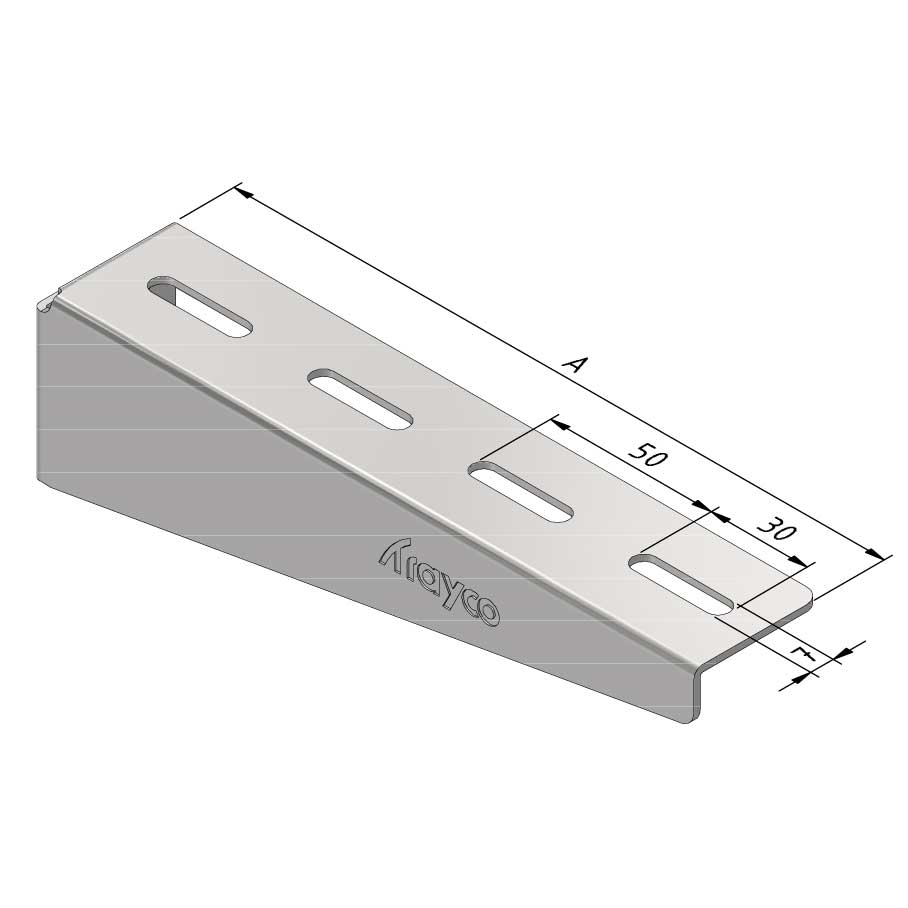
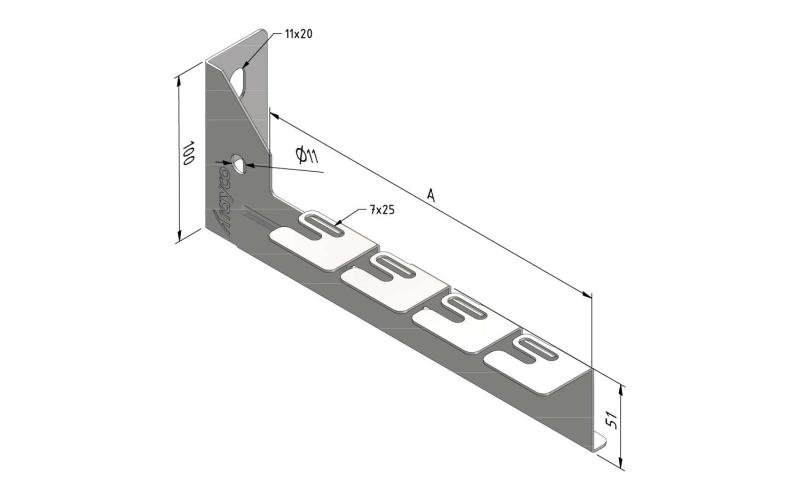
Wall Bracket
SS-WB11
Wall Bracket
SS-WB11
| SKU | Article code | Finishing | Dimension A | F (kN) | Packaging | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
19432 |
WB11-0100-SS316 |
SS316
|
106
|
1.1
|
25
|
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19433 |
WB11-0150-SS316 |
SS316
|
156
|
1.1
|
30
|
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19434 |
WB11-0200-SS316 |
SS316
|
206
|
1.1
|
30
|
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19435 |
WB11-0300-SS316 |
SS316
|
306
|
1.1
|
40
|
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19436 |
WB11-0400-SS316 |
SS316
|
406
|
1.1
|
30
|
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19558 |
WB11-0500-SS316 |
SS316
|
506
|
1.5
|
10
|
Default
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
|
|
19562 |
WB11-0600-SS316 |
SS316
|
606
|
1.5
|
10
|
Default
|
|
|||
Workload
Additional information
Finishing
Stainless steel (1.4404) AISI 316L
Adding chrome(±13%) to the iron creates a certain sheen and the metal becomes more corrosion-resistant. The advantage, compared to other protective coatings, is that it’s not a one-off surface protection. In fact, the stainless steel (or rather the chrome) forms a thin, invisible layer of chromium oxide whenever it comes into contact with oxygen: the oxide film. This thin layer protects the underlying stainless steel from further corrosion (oxidation). If the oxide flm suffers damage, then the underlying stainless steel will be re-exposed to the oxygen in the air and the protective coat will re-form. In this way, it repairs itself whenever it gets damaged. In certain circumstances or if the protective oxide flm is damaged, the corrosion can be locally quite rapid. This is caused, among other things, by chlorides or other materials (usually iron) that lodge in the surface. Due to this contamination, pitting corrosion may occur that corrodes the stainless steel (SS). That’s why, after the processing, the SS is treated, to remove all possible impurities. This is the `pickling’ stage. As mentioned above, a passive chromium layer protects the steel and repairs itself automatically. There are, however, circumstances in which this repair does not happen. Certain process steps may disrupt the balance in such a way that the passive state disappears and an active layer is formed. This could occur during process steps such as welding, bending or machining (with removal of metal or wood), giving rise to oxygen-poor pockets and the repair fails to materialise. Consequently, the rustproof properties are lost and, if exposed to gases or liquids, corrosion will occur. To remedy this situation, the active layer is re-converted to a passive layer (this process is known as passivating). In is usually desirable to degrease the semi-fnished products and, after that, to pickle them in a mixture of nitric acid (HNO3) and hydrogen fluoride (HF), in order to remove impurities from the metal surface. This may be necessary on welded surfaces or on rotated objects for which a coolant is used. |
|||||||||||
No results
No results were found for your current search