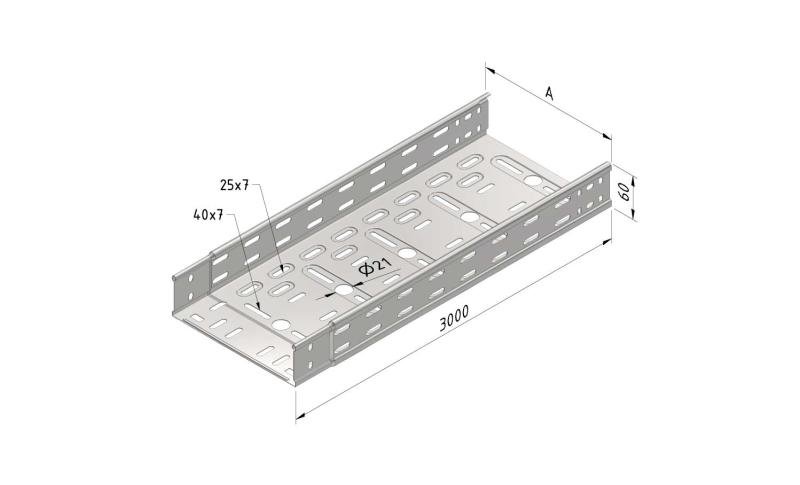
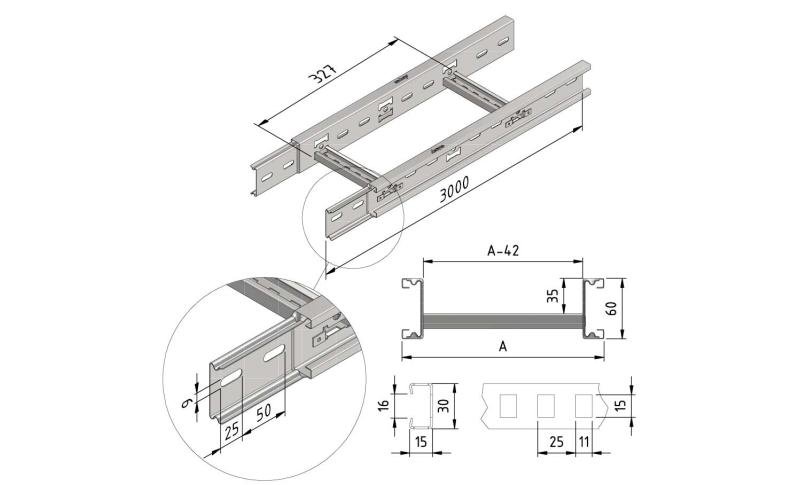
Roundheadbolt and Nut
SS-BN
Roundheadbolt and Nut
SS-BN
| SKU | Article code | Finishing | Packaging | |||
|---|---|---|---|---|---|---|
|
|
12787 |
BN06-10-SS316 |
SS316
|
200
|
Default
|
|
|
|
14044 |
BN06-20-SS316 |
SS316
|
200
|
Default
|
|
|
|
18299 |
BN08-16-SS316 |
SS316
|
200
|
Default
|
|