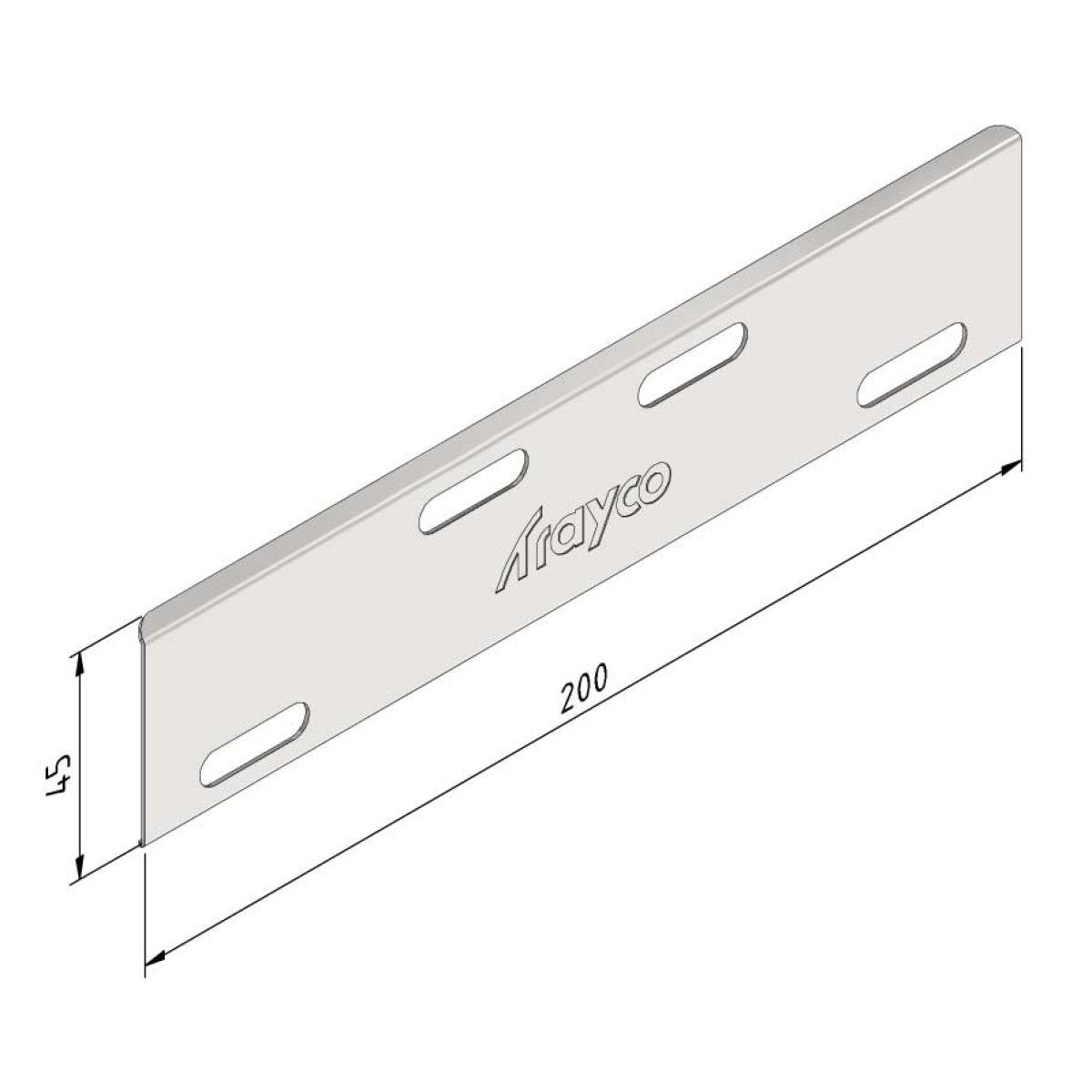
Cable Tray Joint
SS-CT-J
Cable Tray Joint
SS-CT-J
| SKU | Article code | Finishing | Packaging | |||
|---|---|---|---|---|---|---|
|
|
13422 |
CT60-J-SS316 |
SS316
|
20
|
Default
|
|