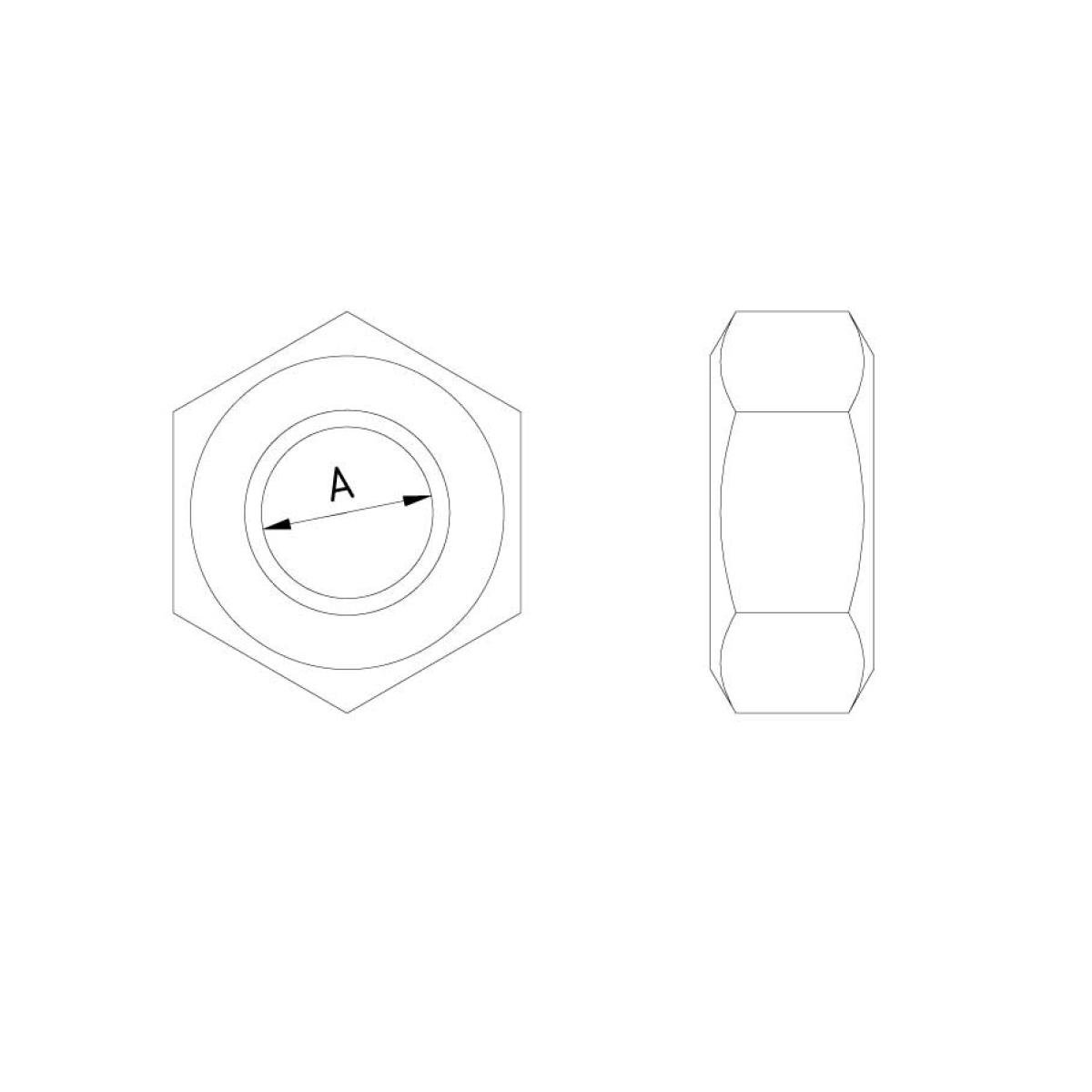
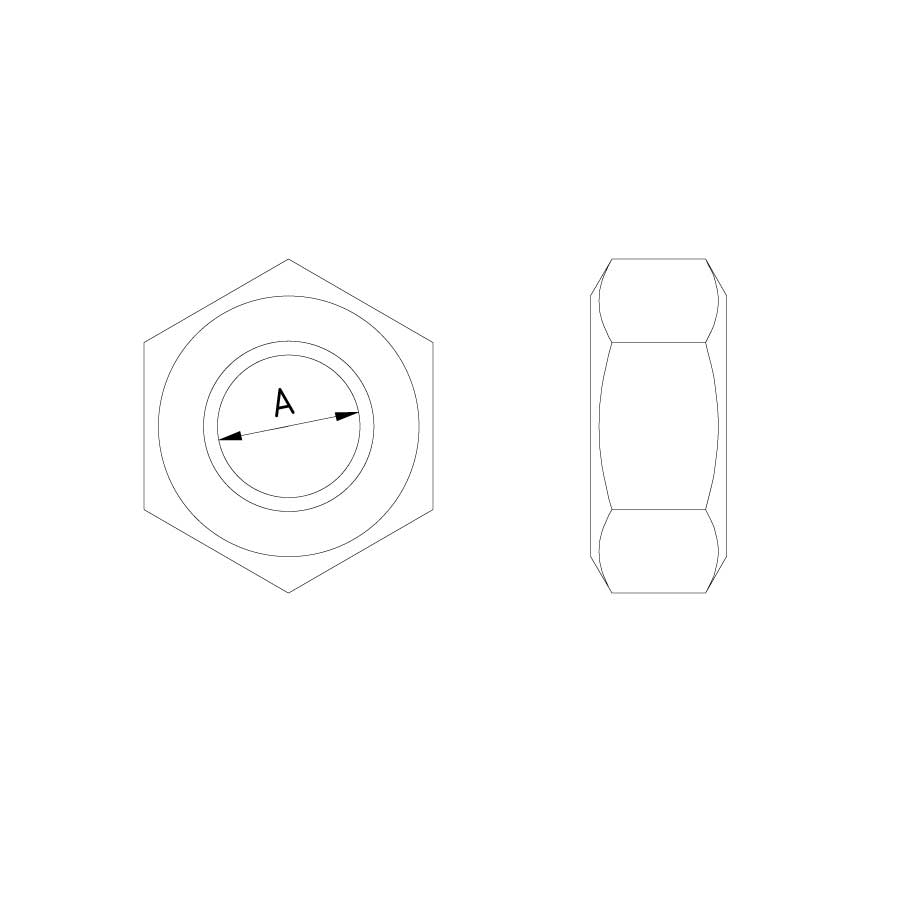
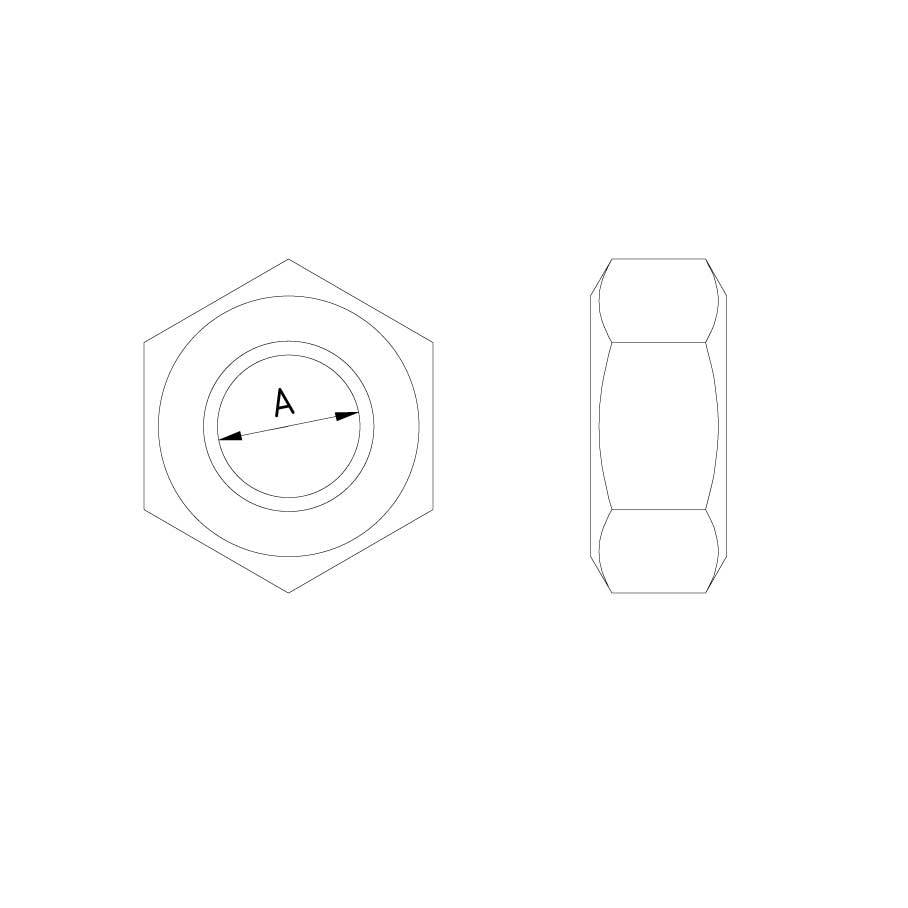
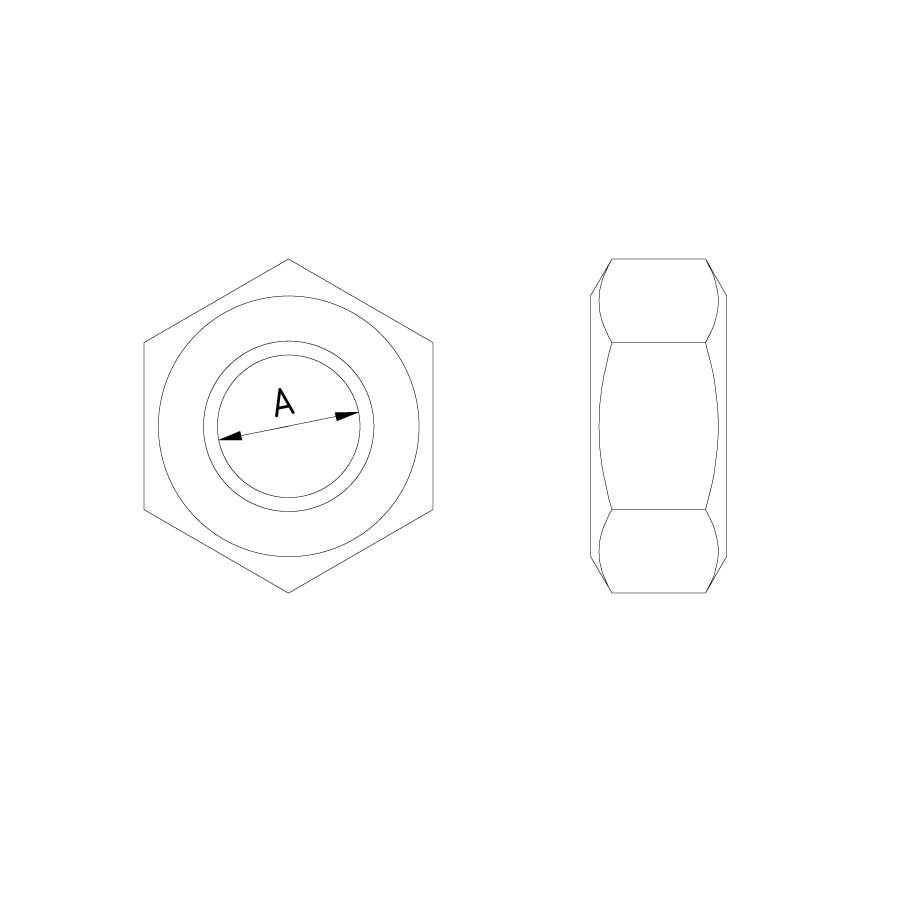
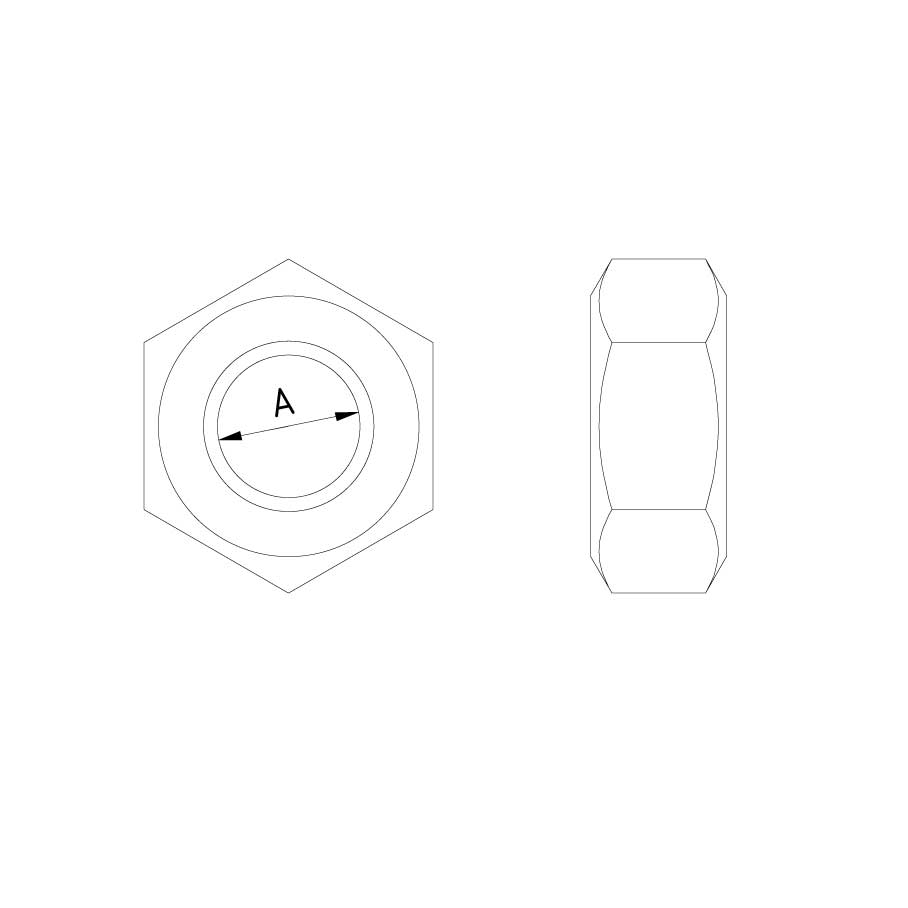
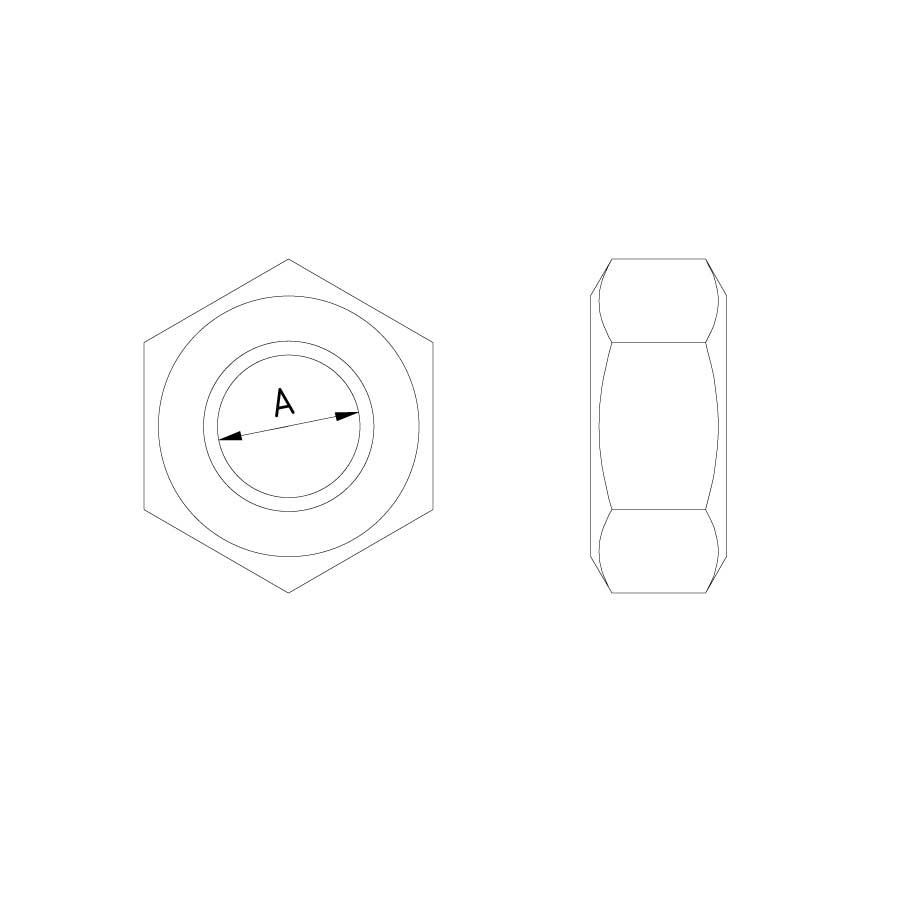
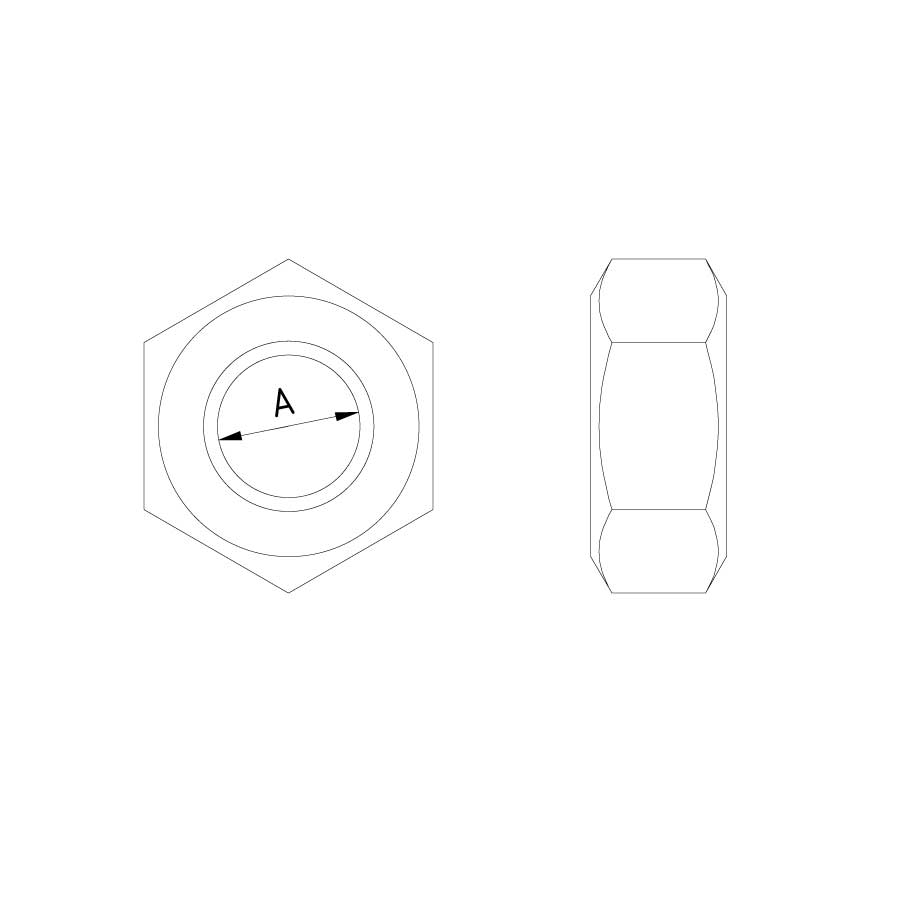
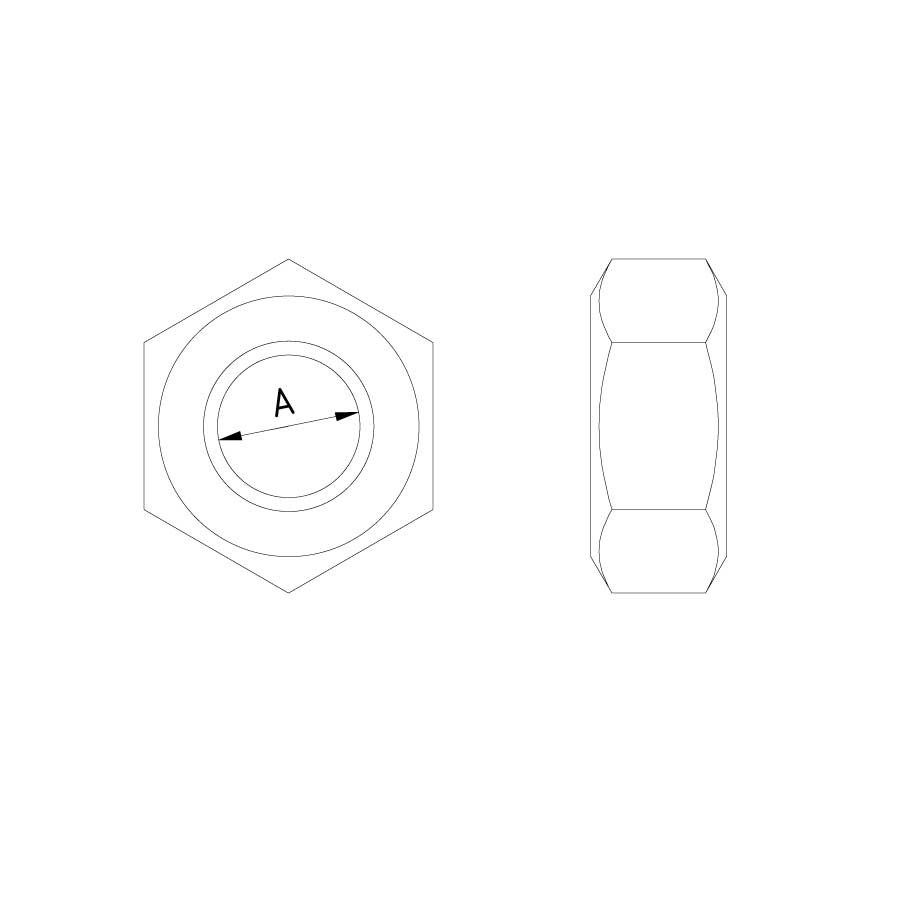
Nut DIN 934
N
Nut DIN 934
N
| SKU | Article code | Finishing | Packaging | |||
|---|---|---|---|---|---|---|
|
|
10363 |
N06-EG |
EG
|
100
|
Default
|
|
|
|
10364 |
N08-EG |
EG
|
100
|
Default
|
|
|
|
10365 |
N10-EG |
EG
|
100
|
Default
|
|
|
|
10366 |
N12-EG |
EG
|
100
|
Default
|
|
|
|
10587 |
N06-DG |
DG
|
100
|
Default
|
|
|
|
10588 |
N08-DG |
DG
|
100
|
Default
|
|
|
|
10589 |
N10-DG |
DG
|
100
|
Default
|
|
|
|
10590 |
N12-DG |
DG
|
100
|
Default
|
|