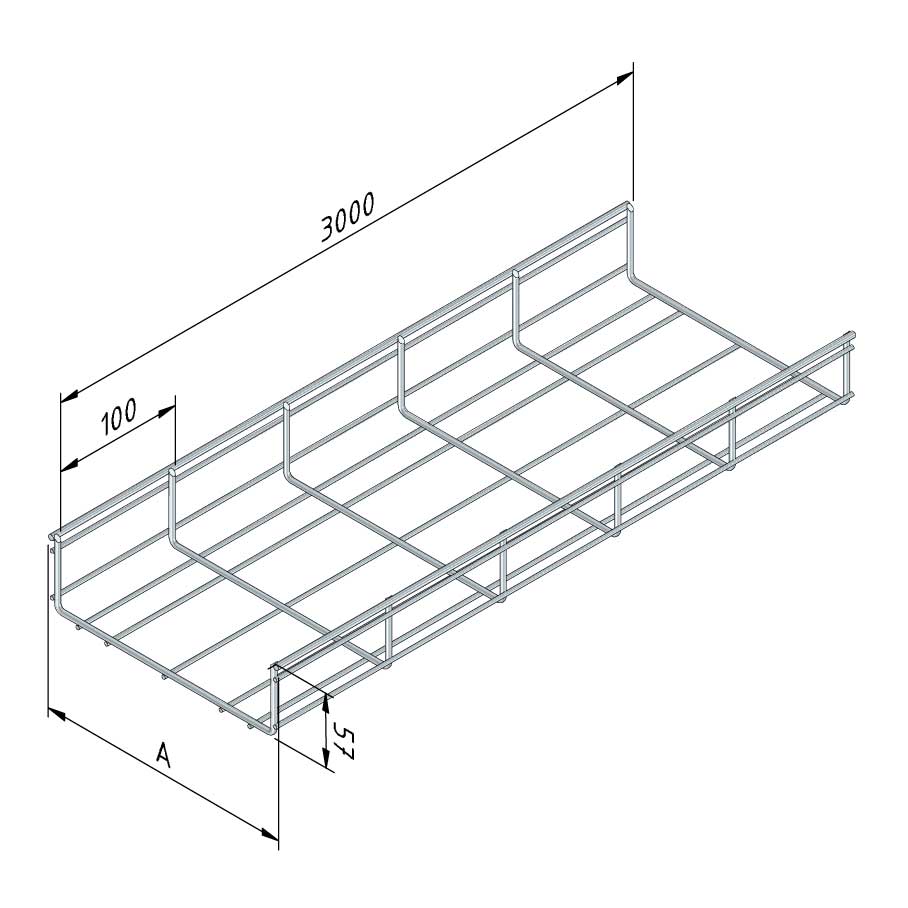
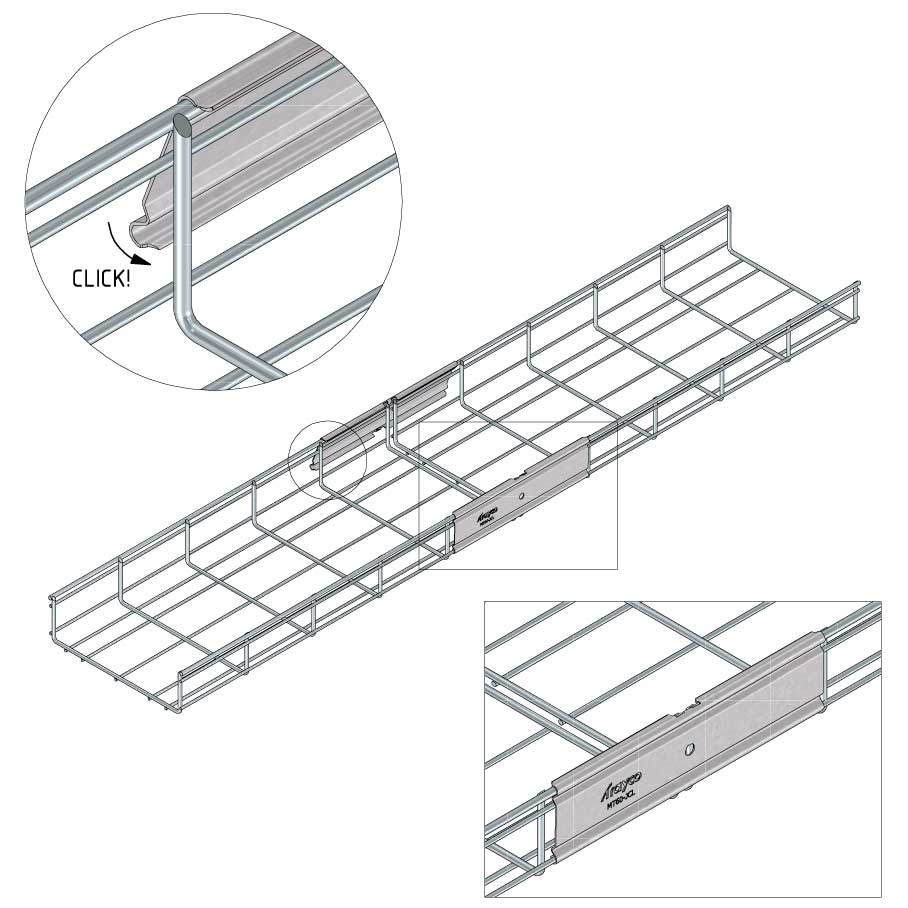
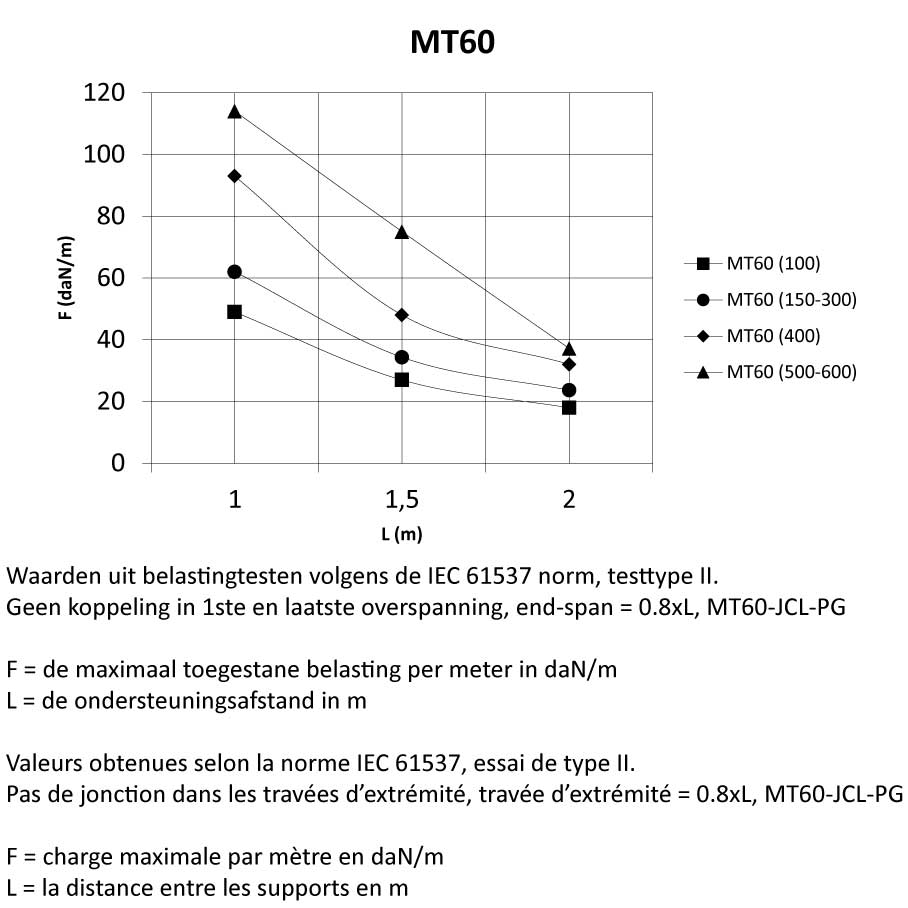
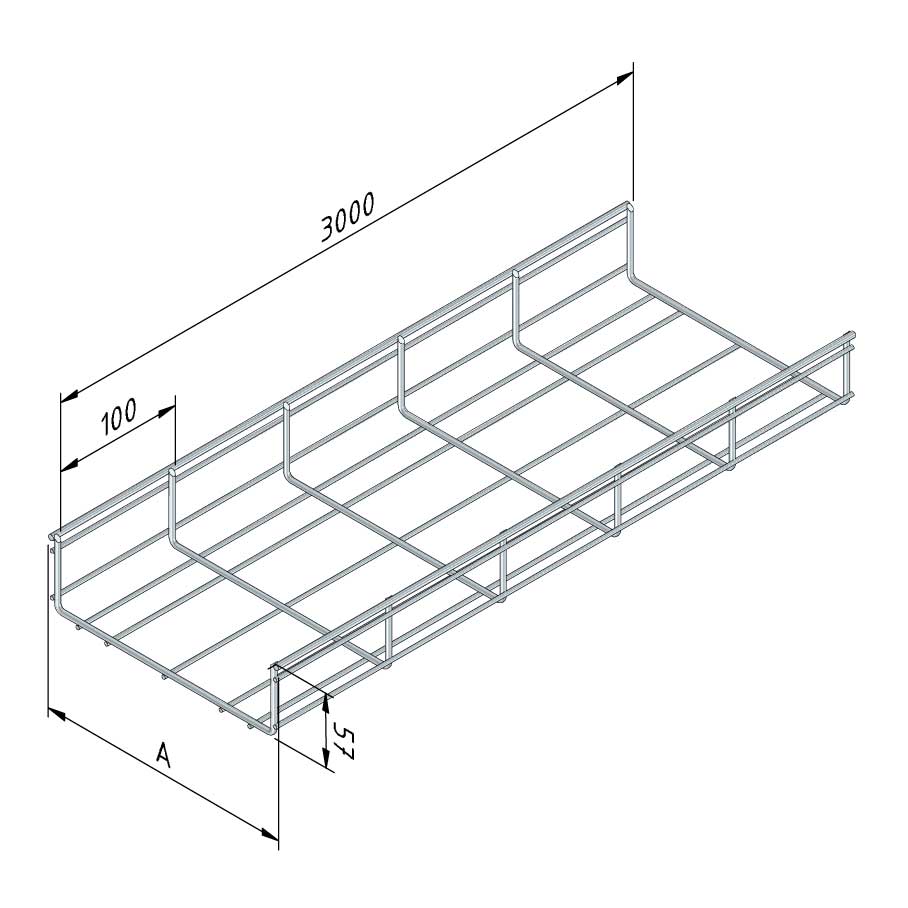
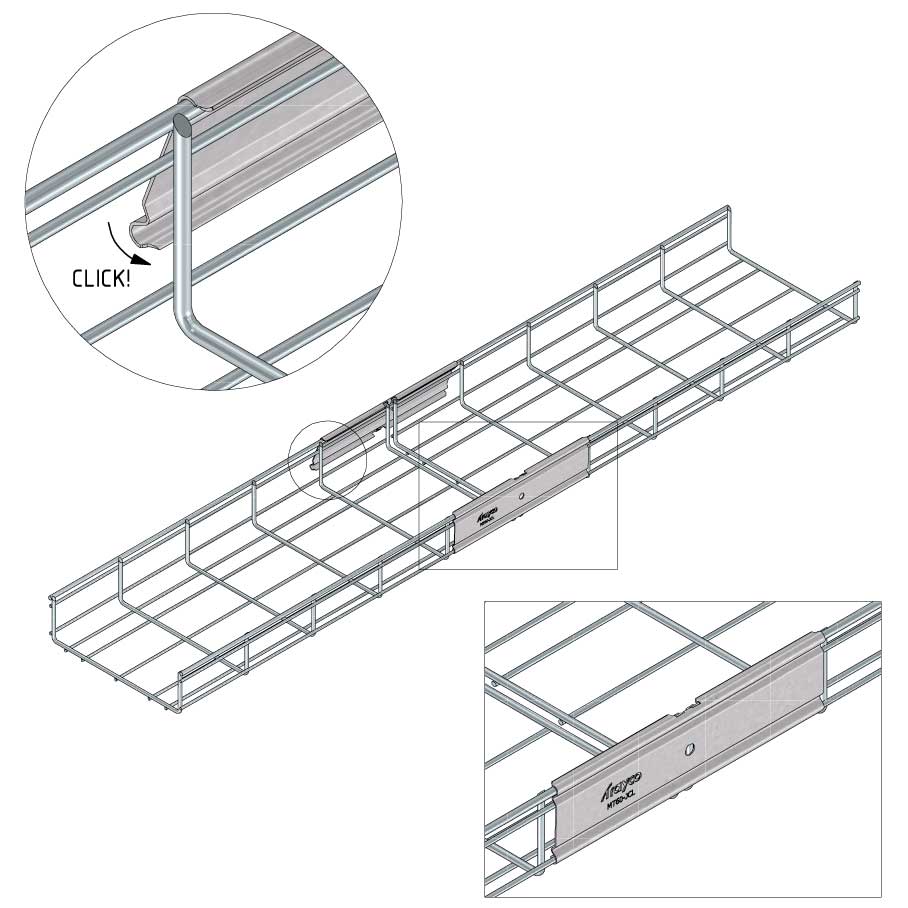
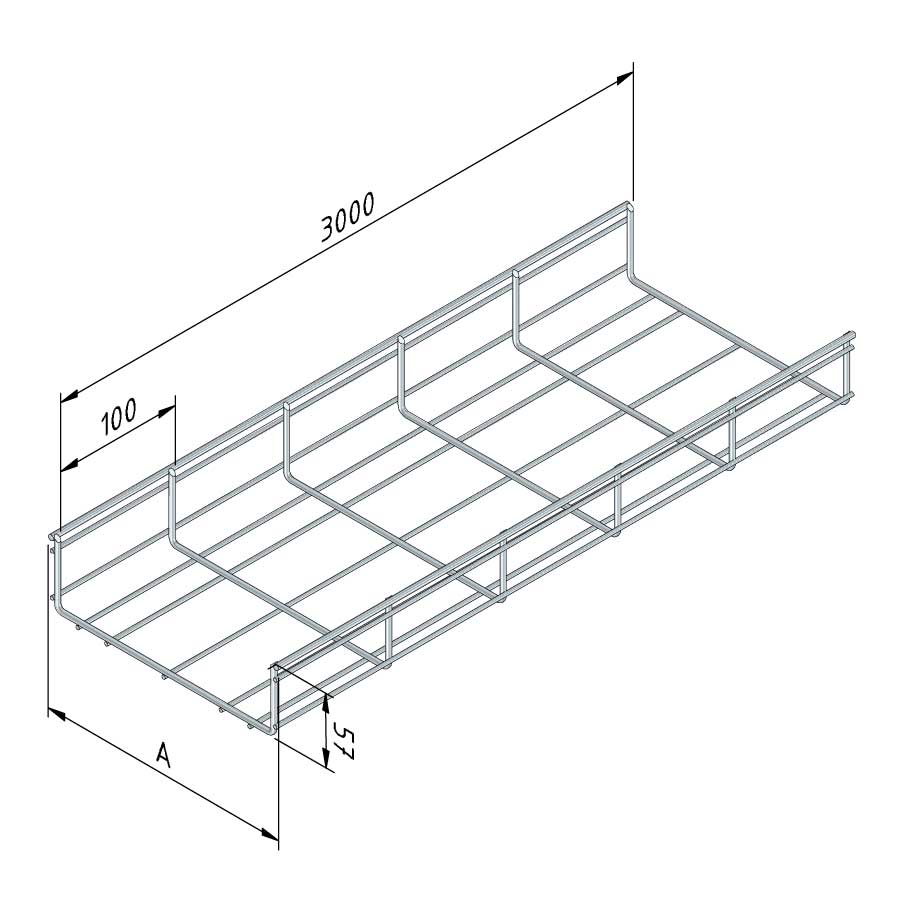
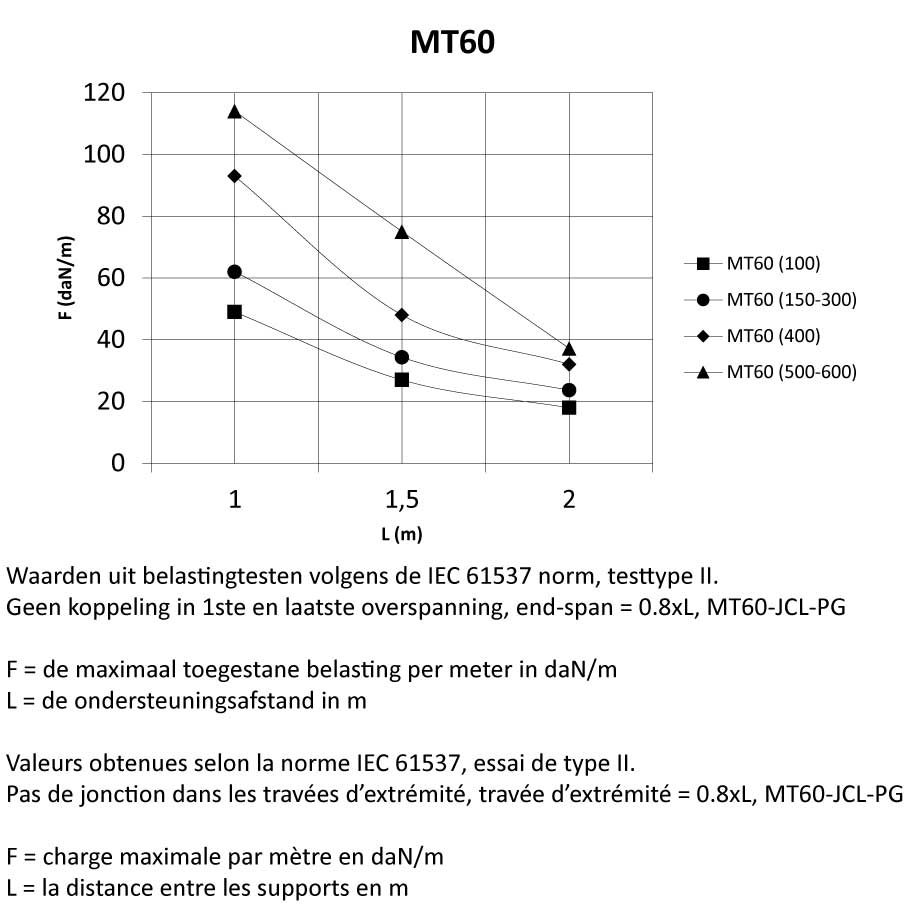
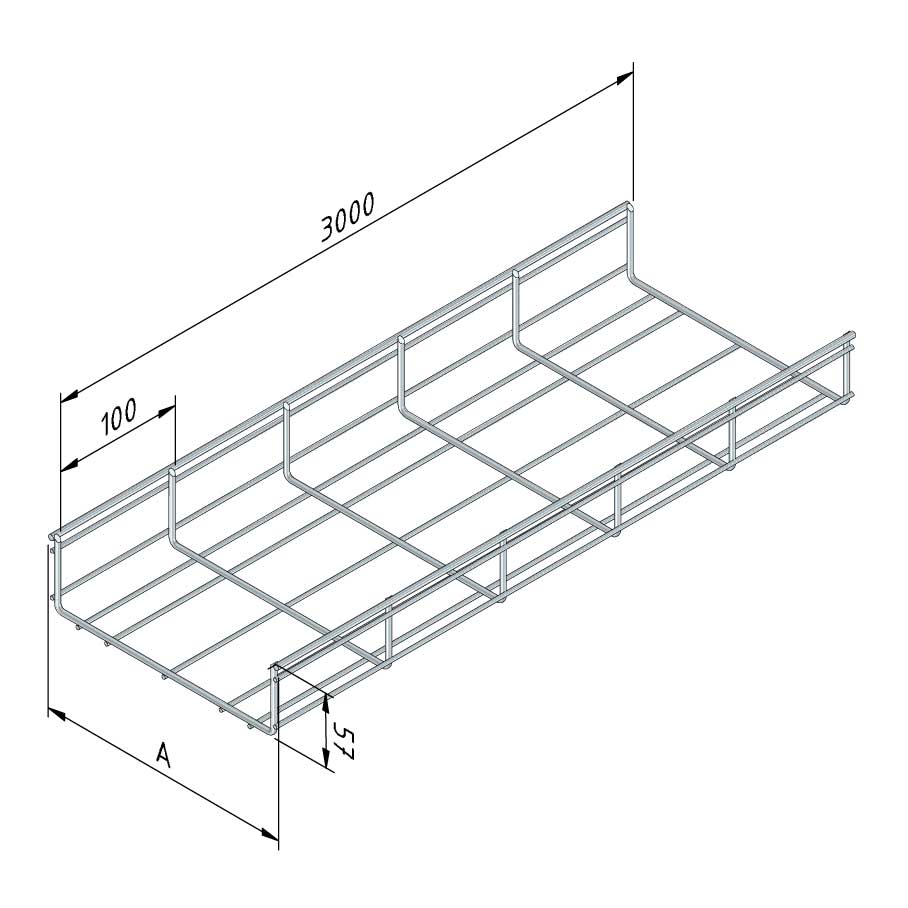
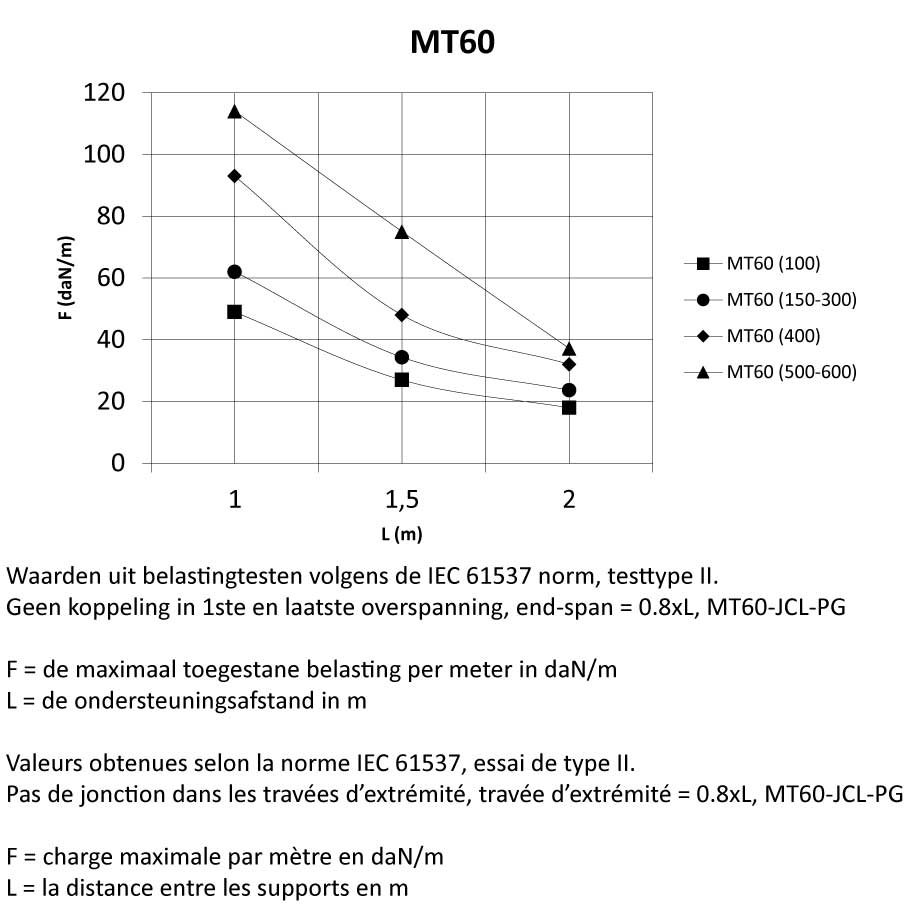
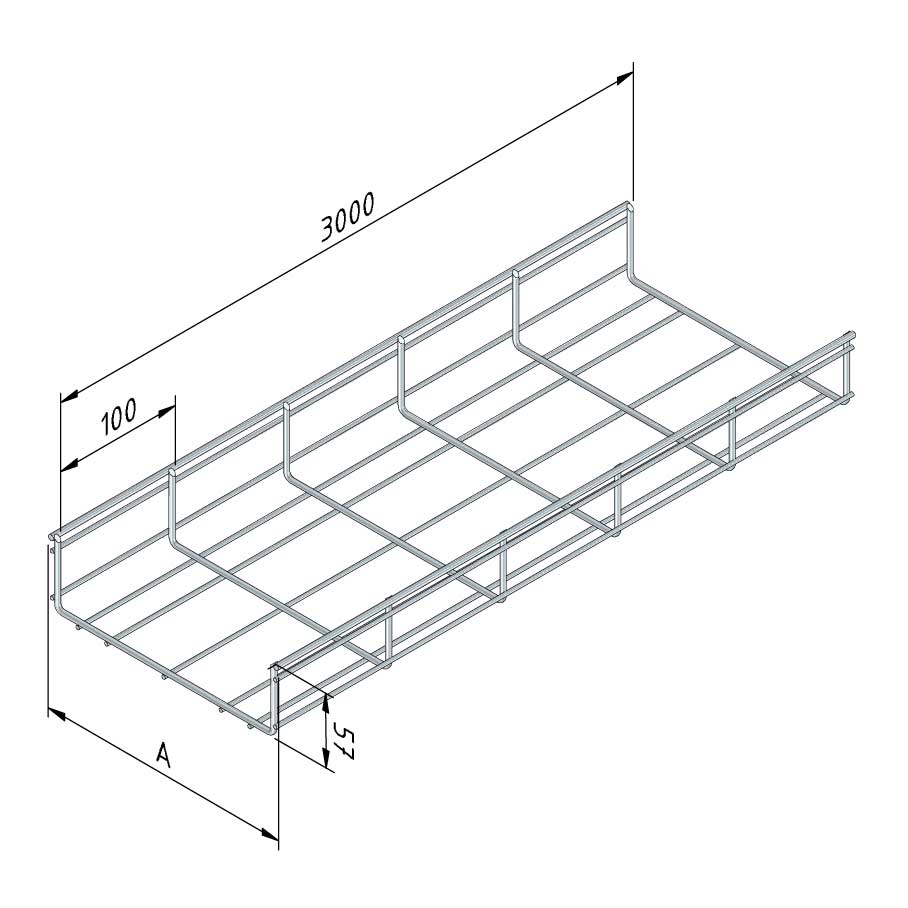
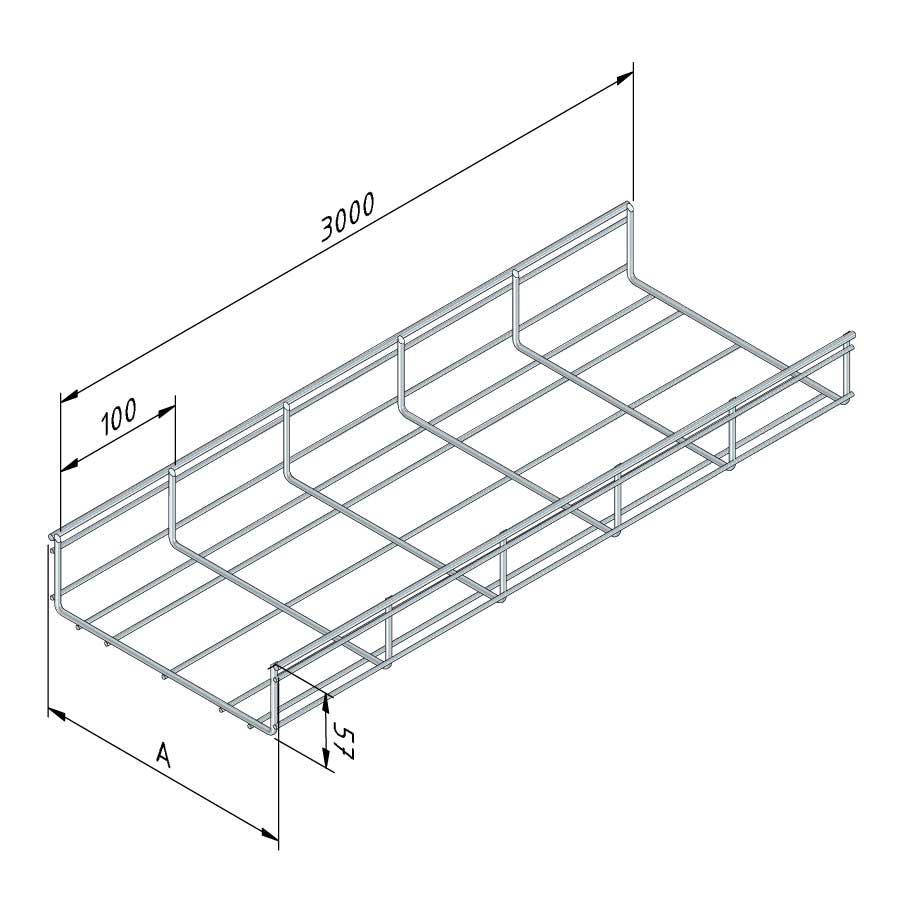
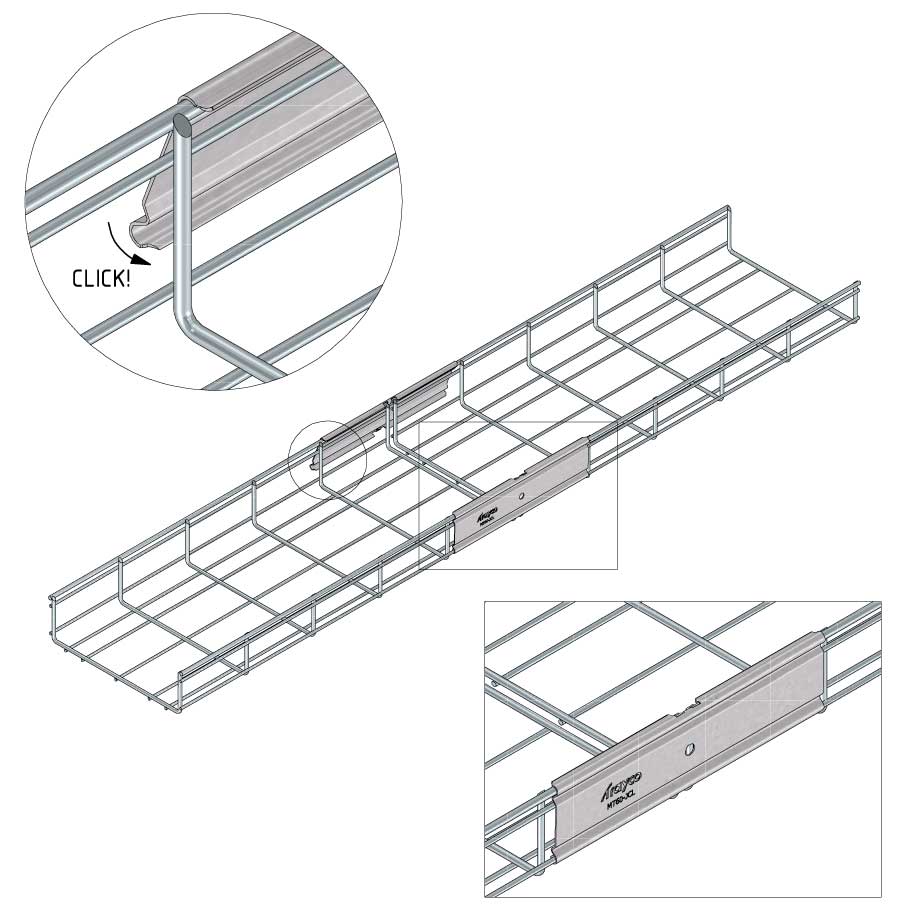
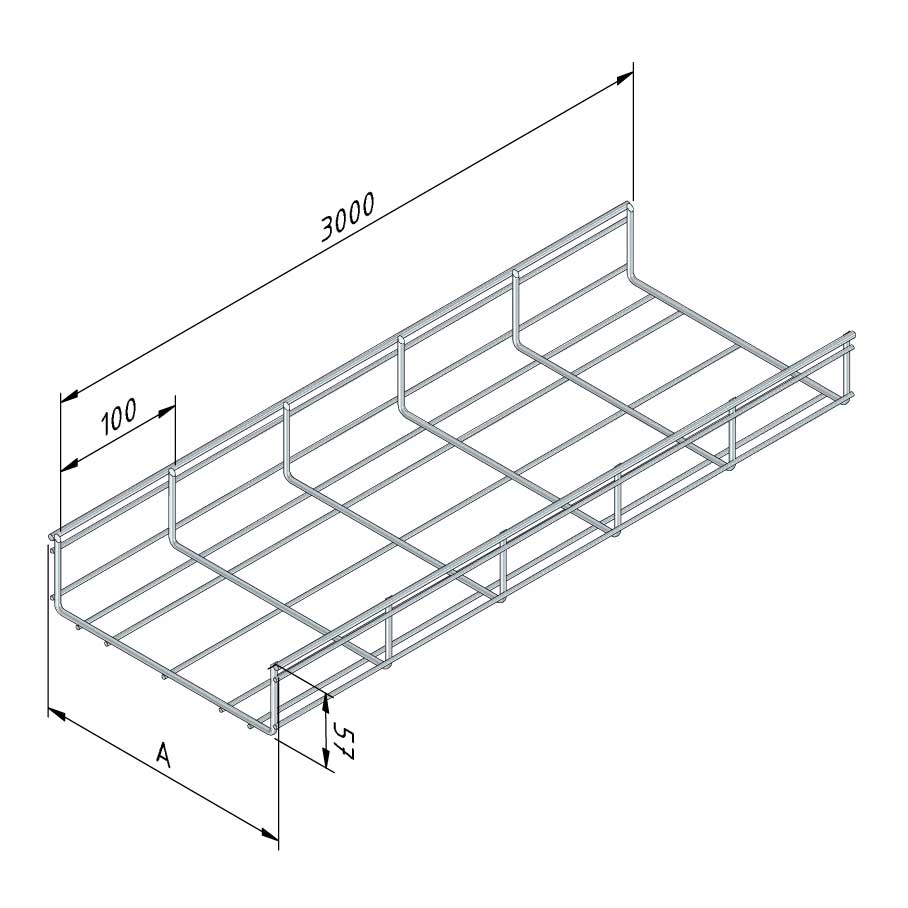
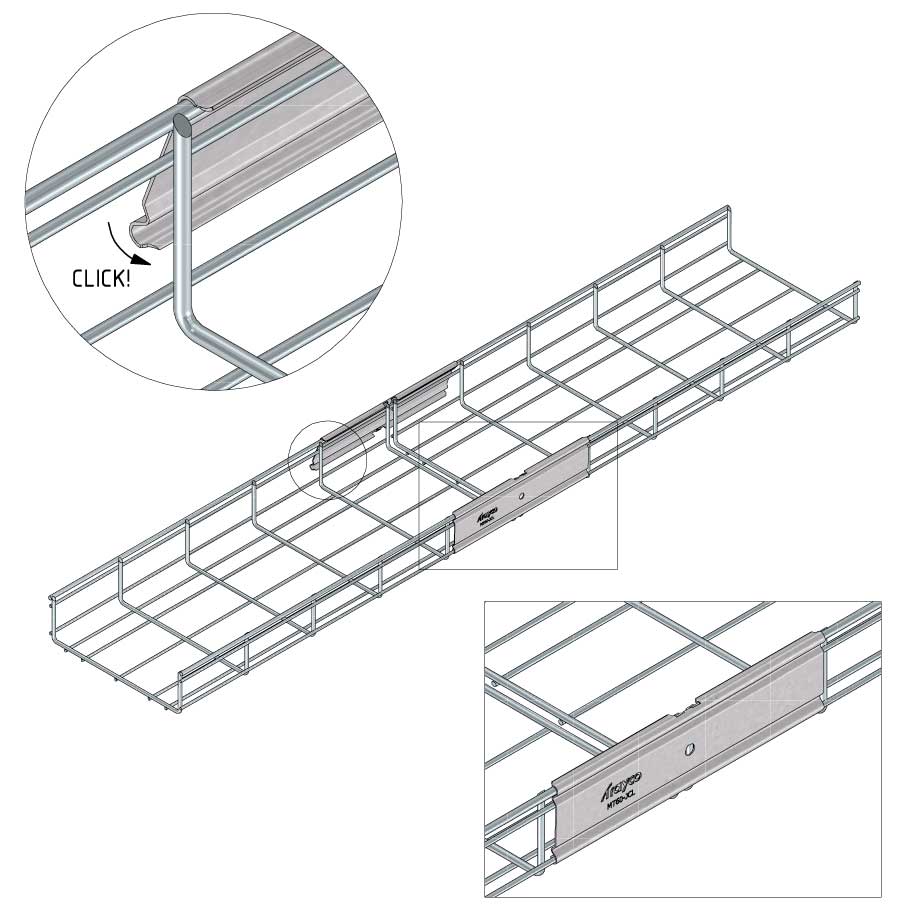
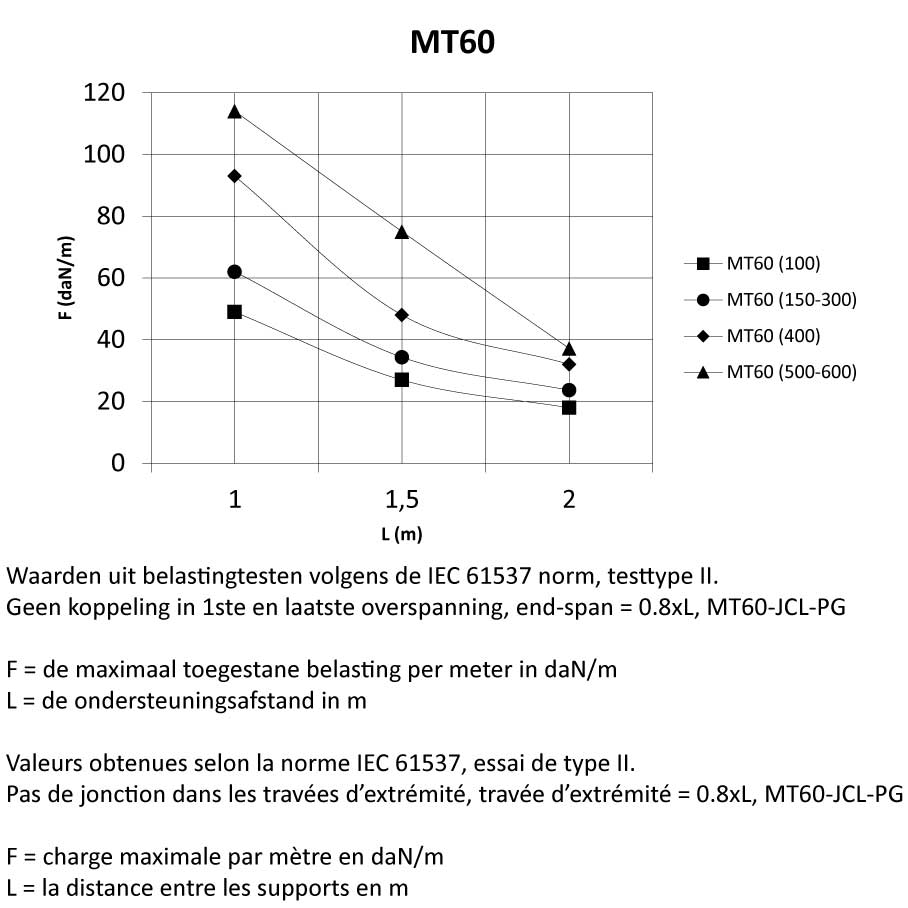
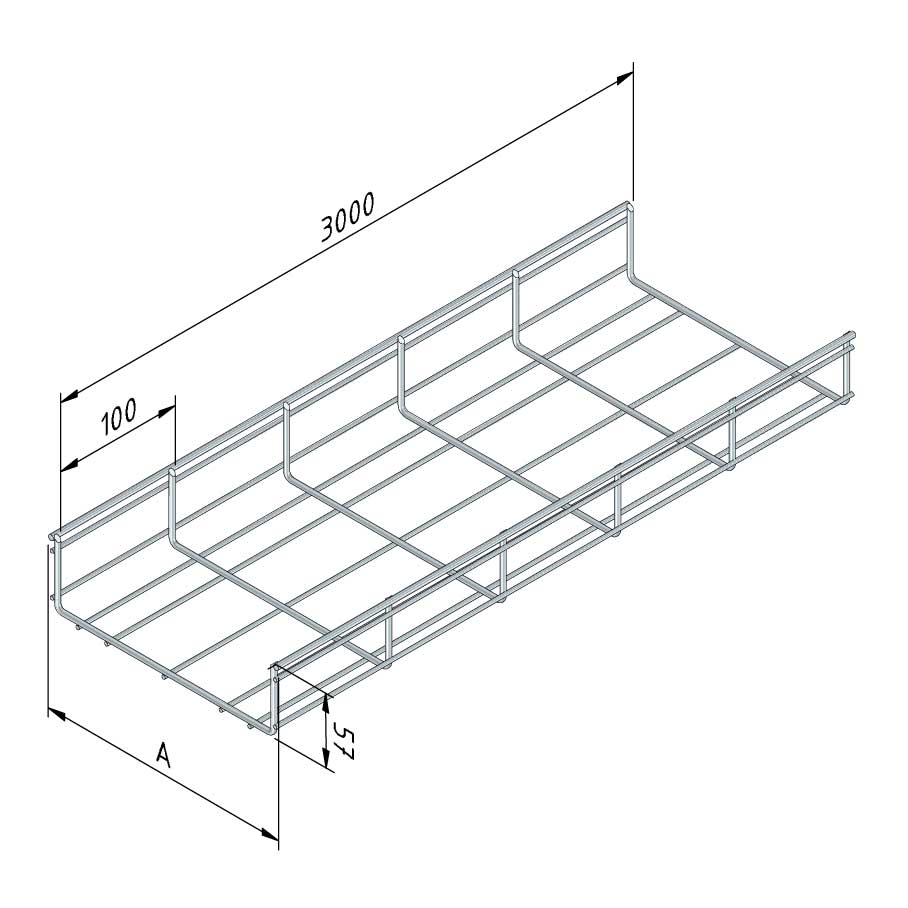
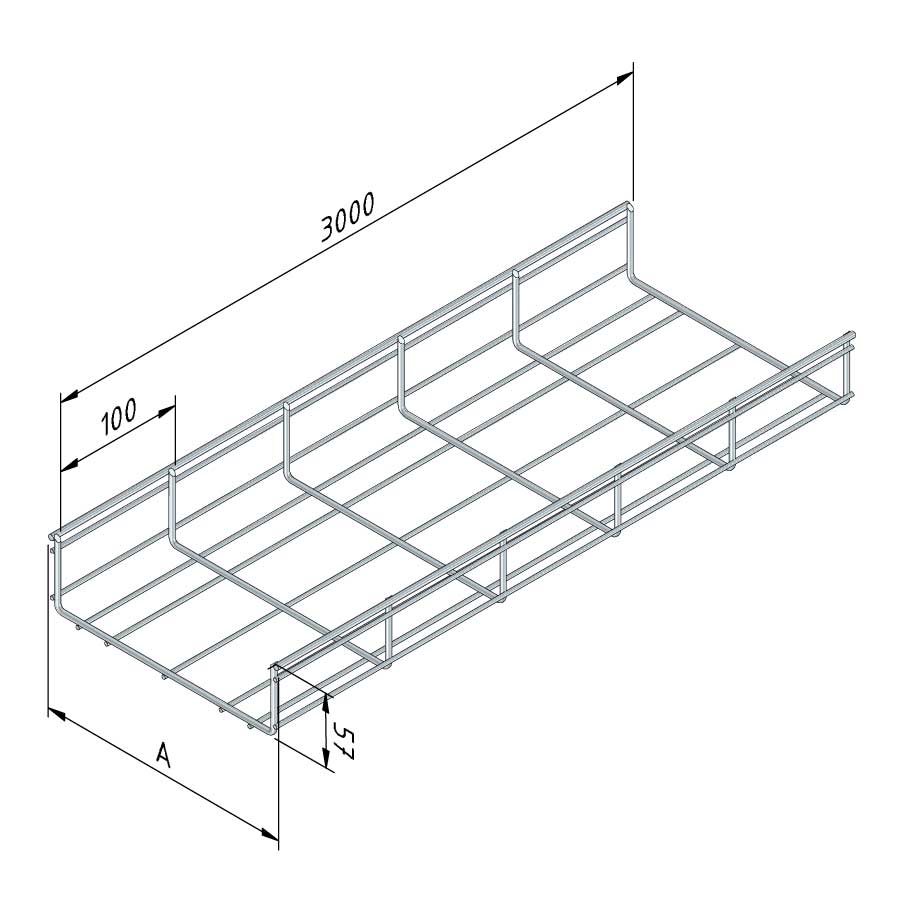
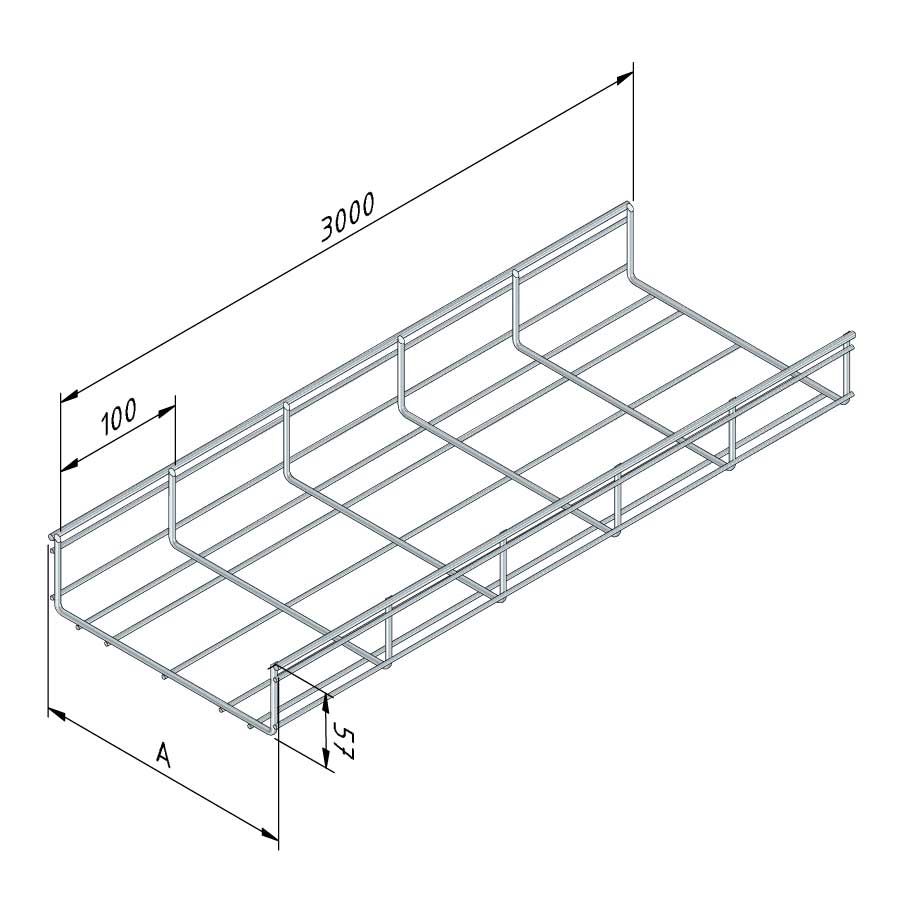
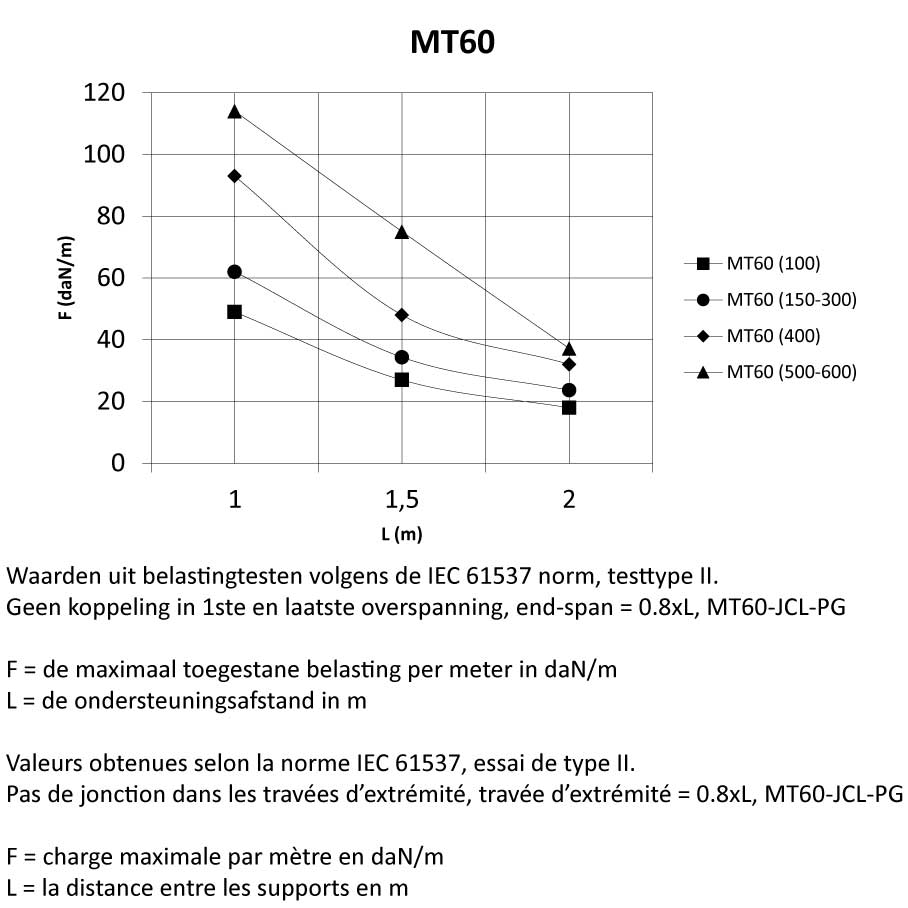
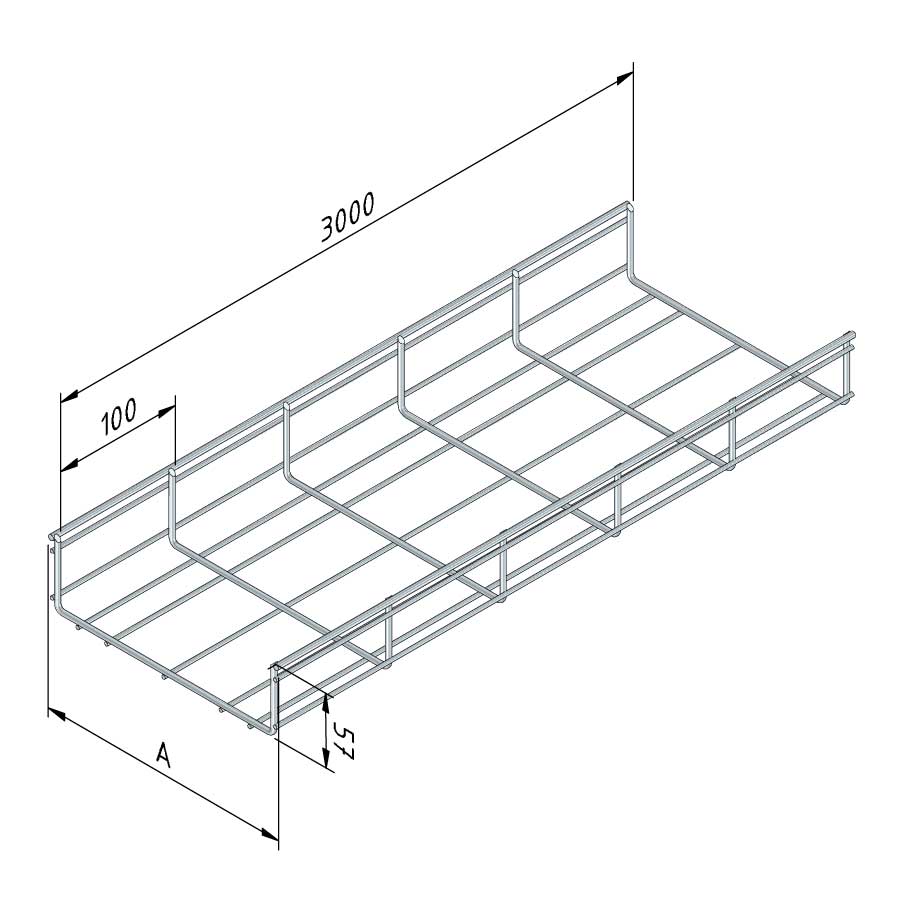
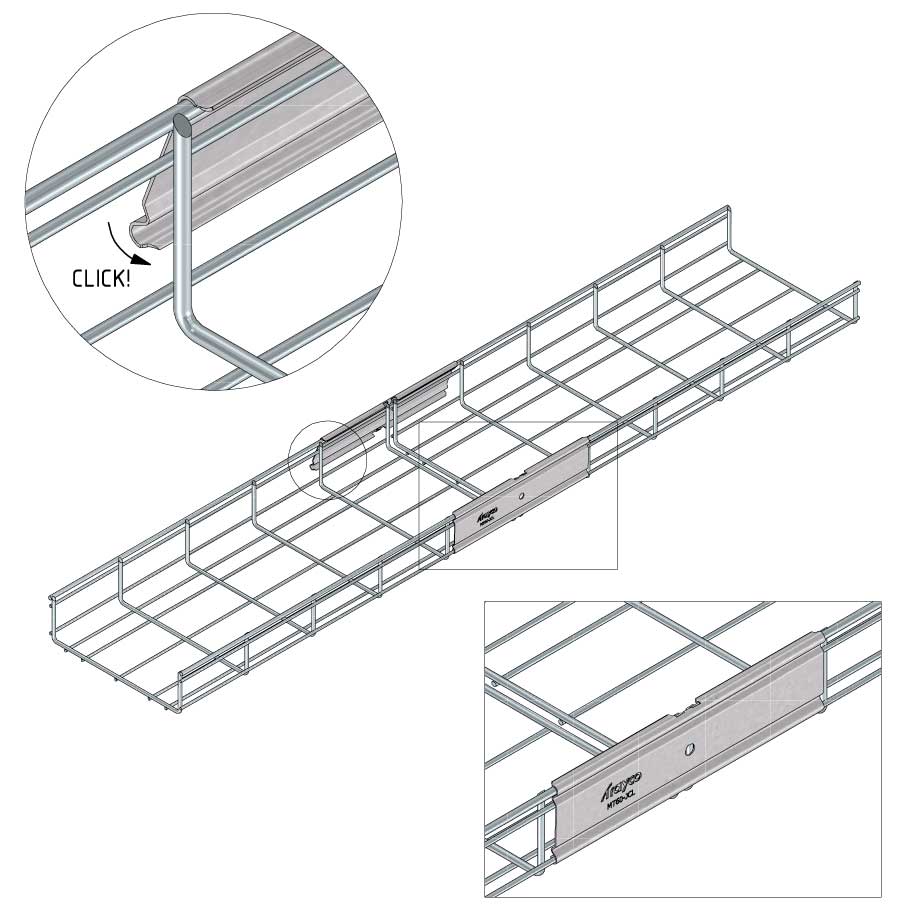
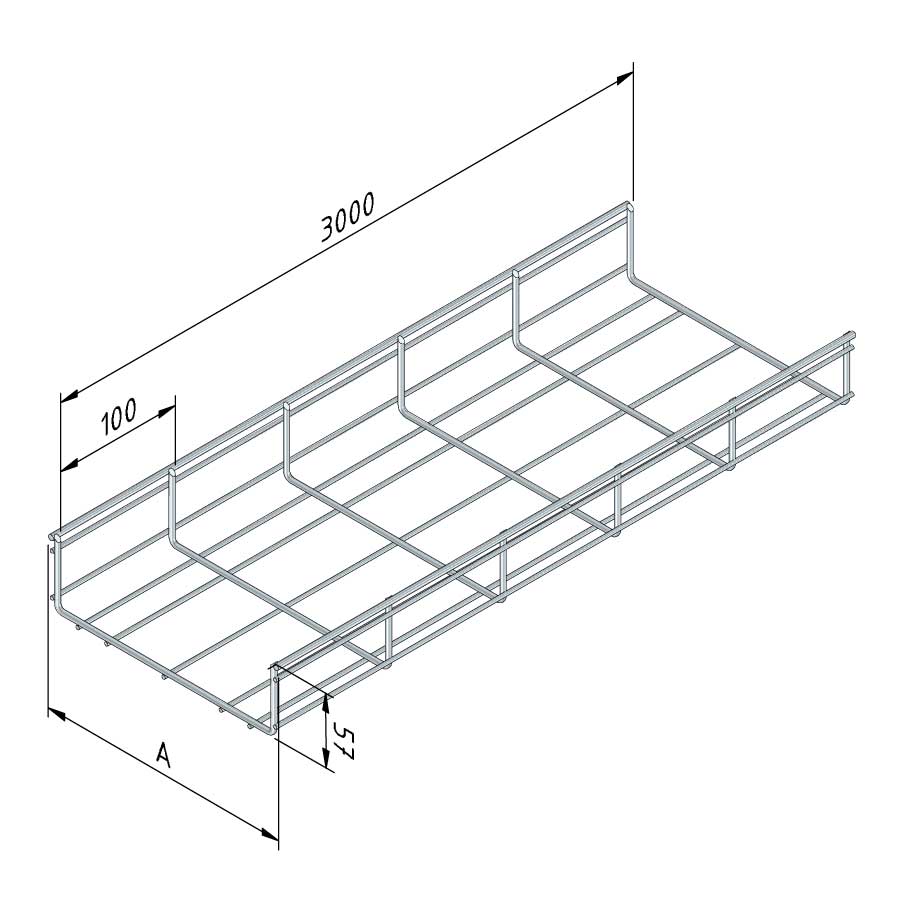
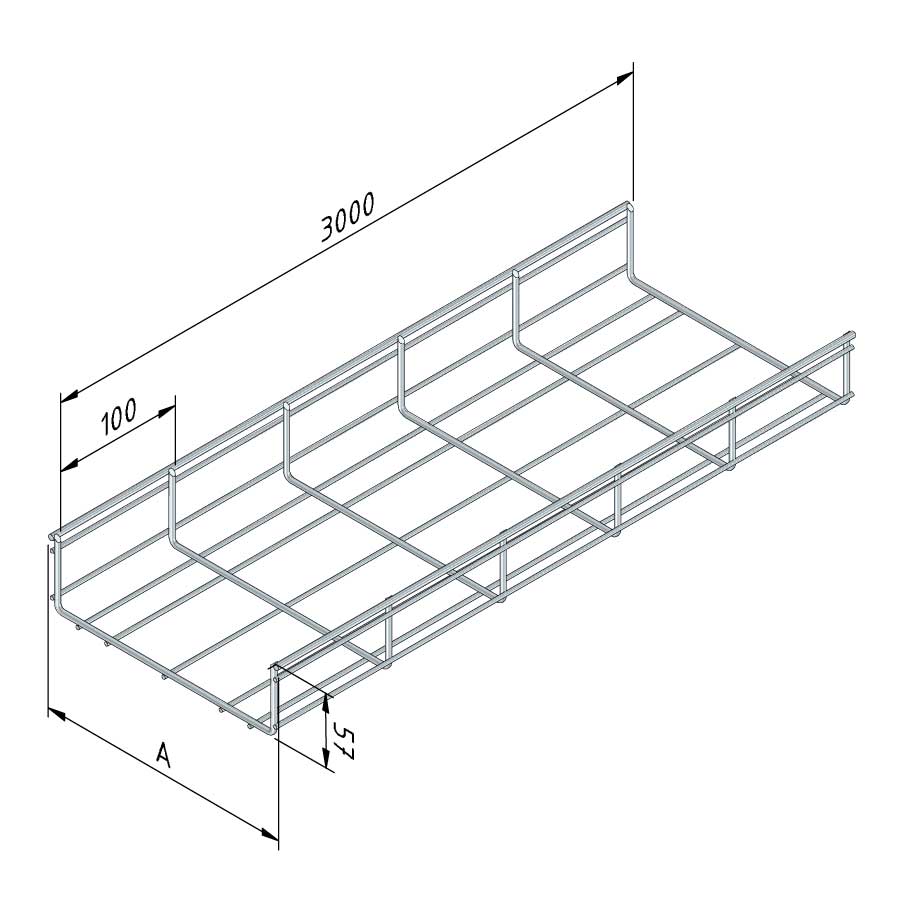
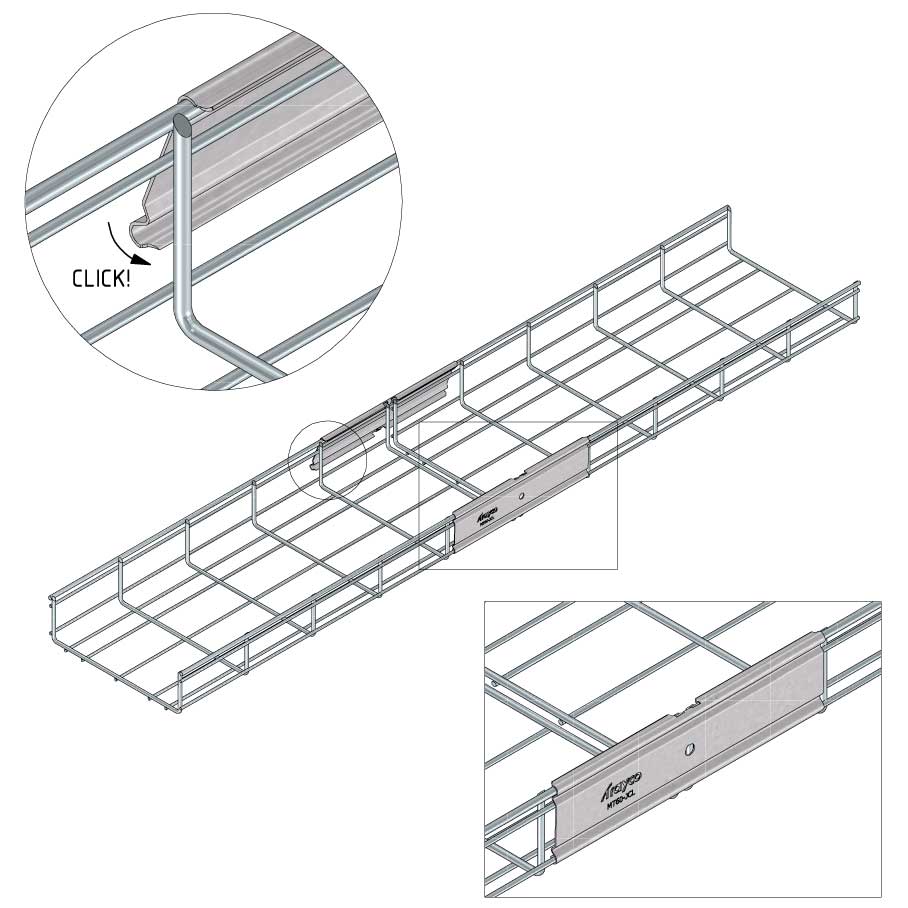
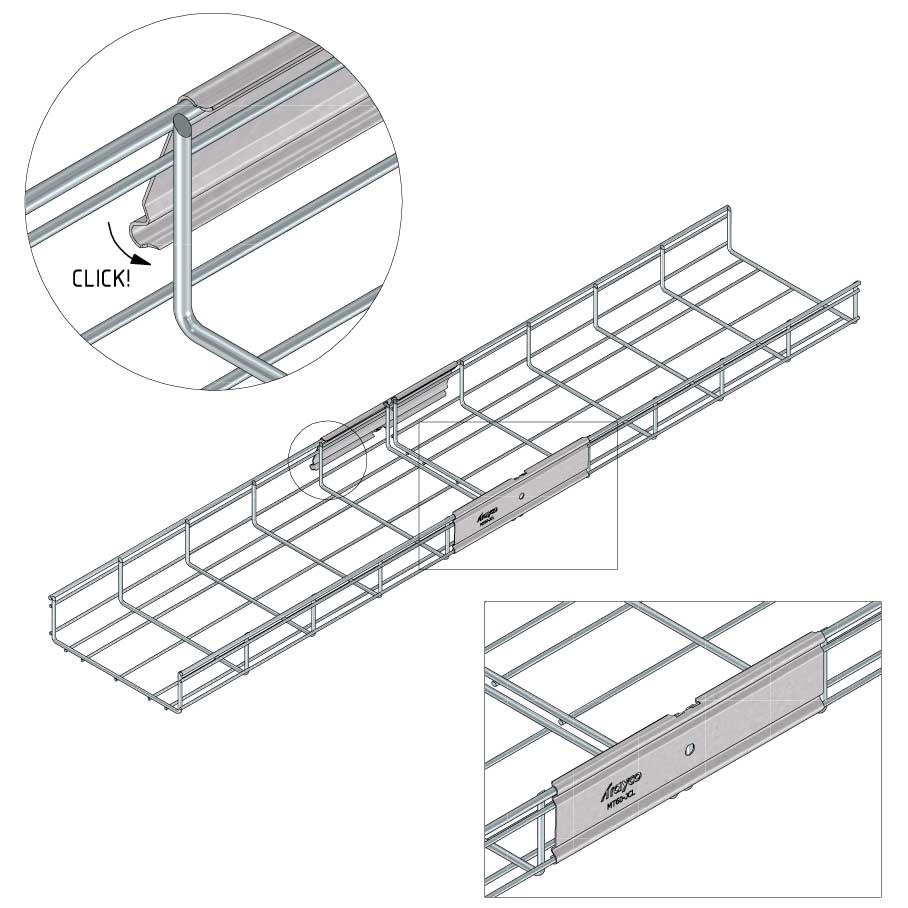
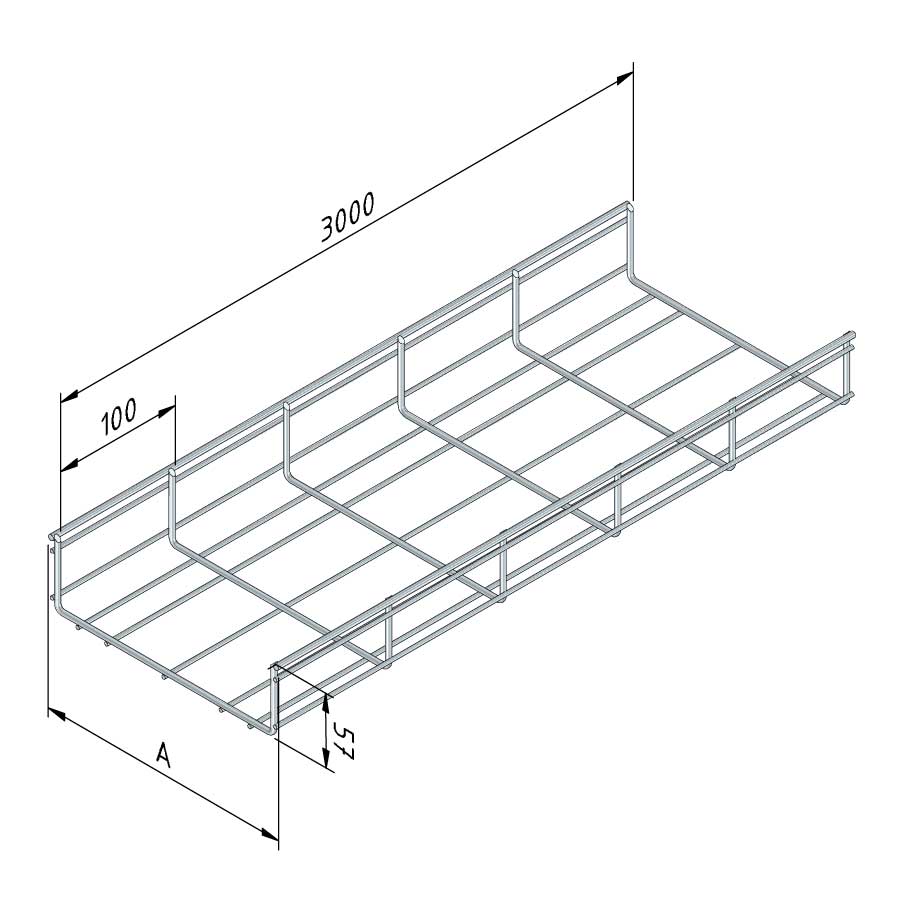
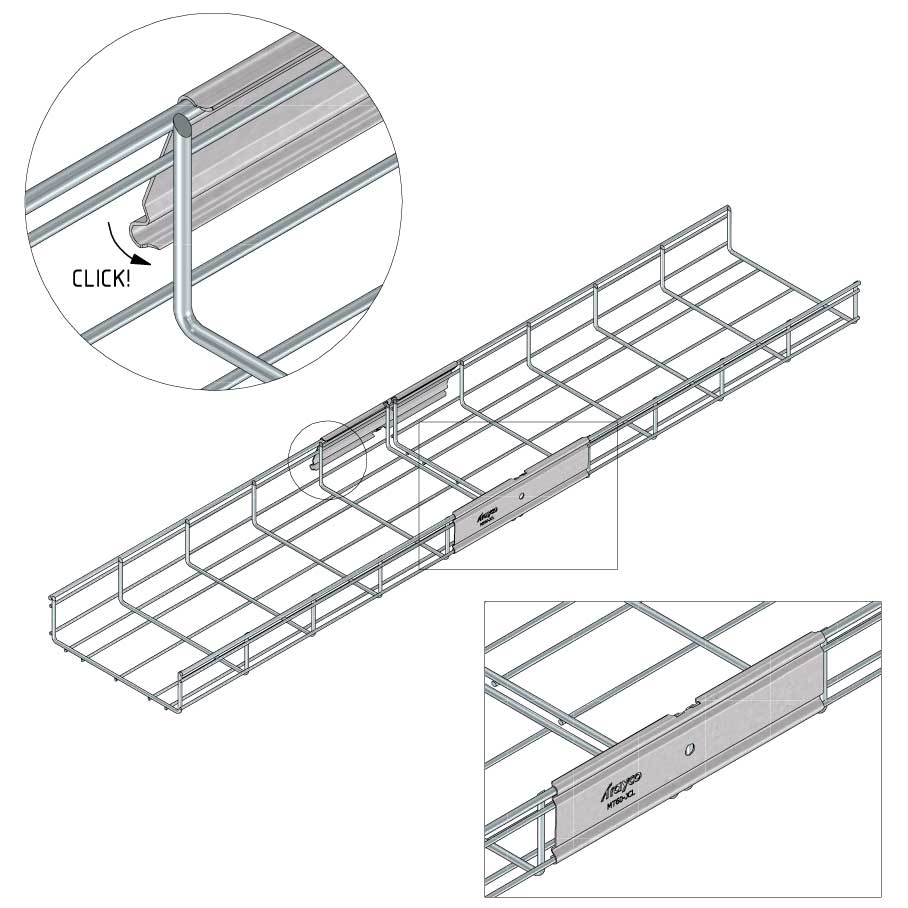
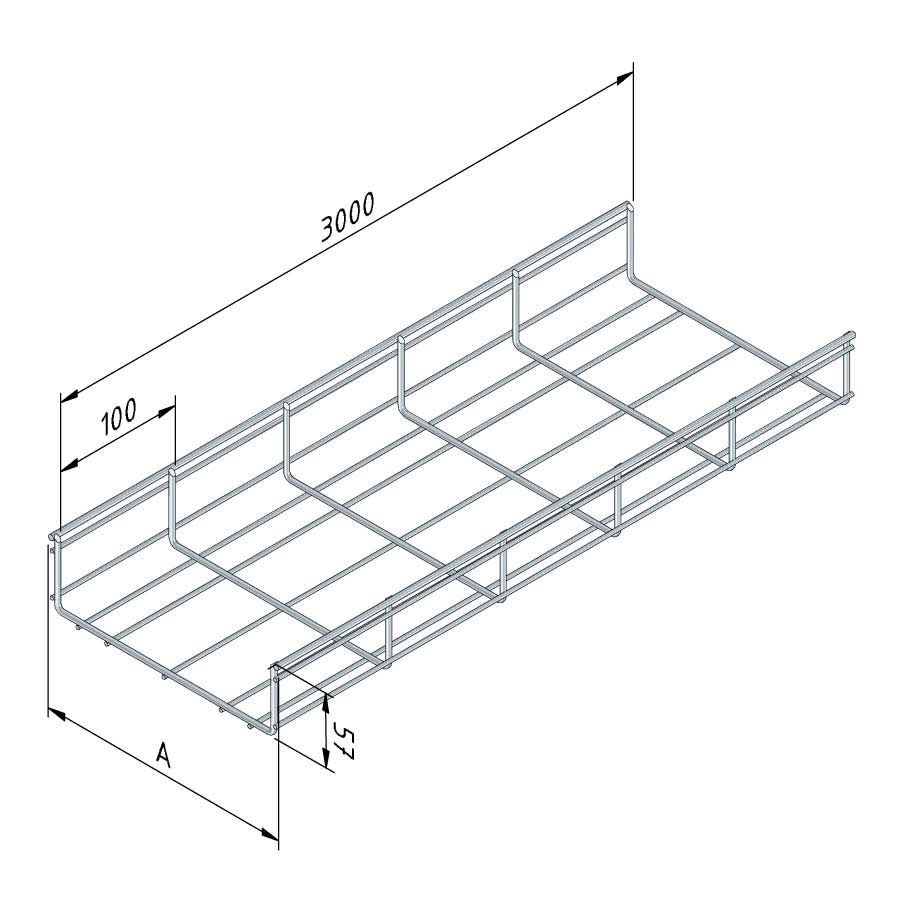
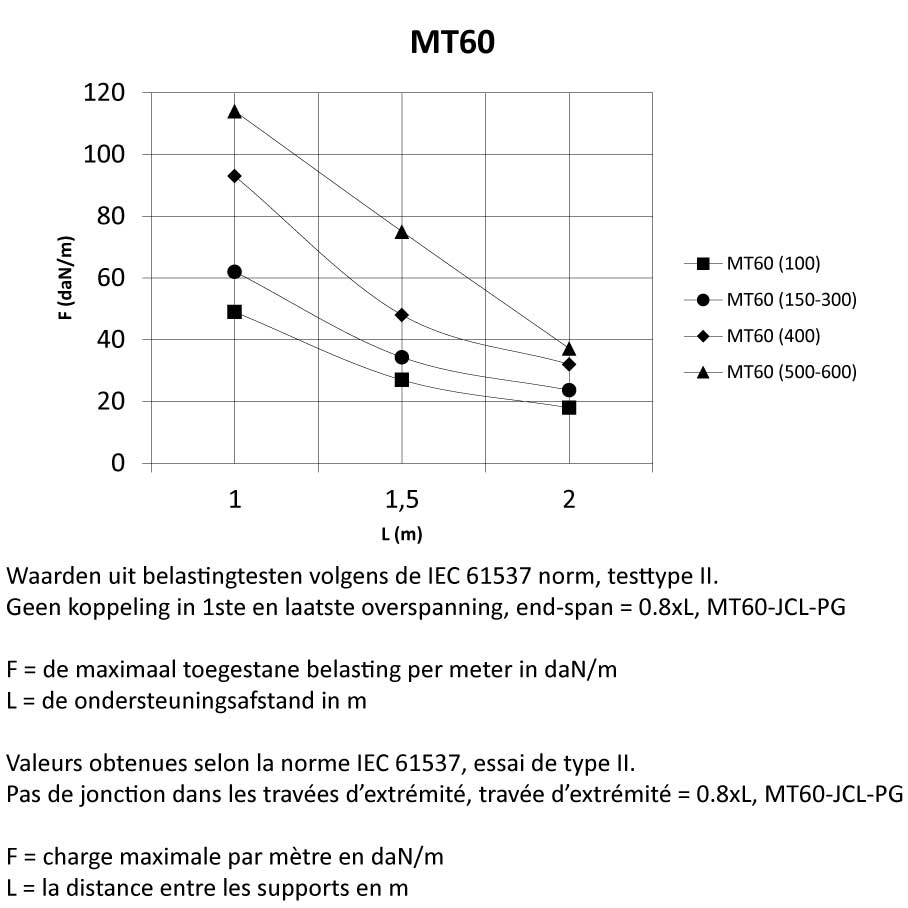
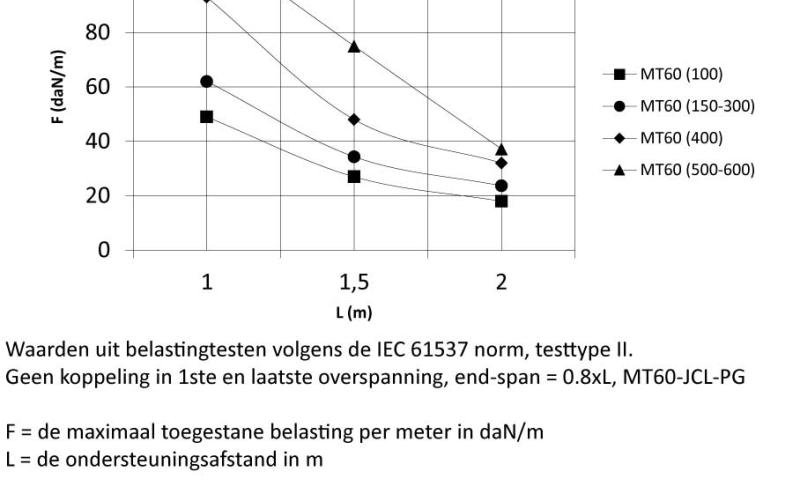
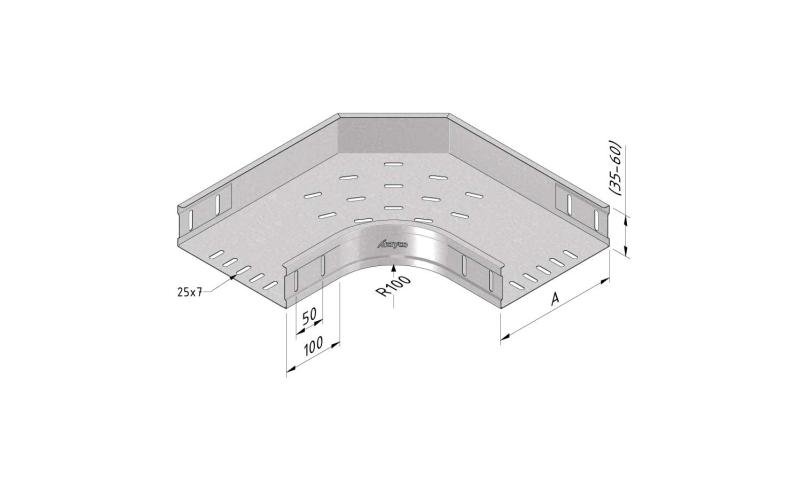
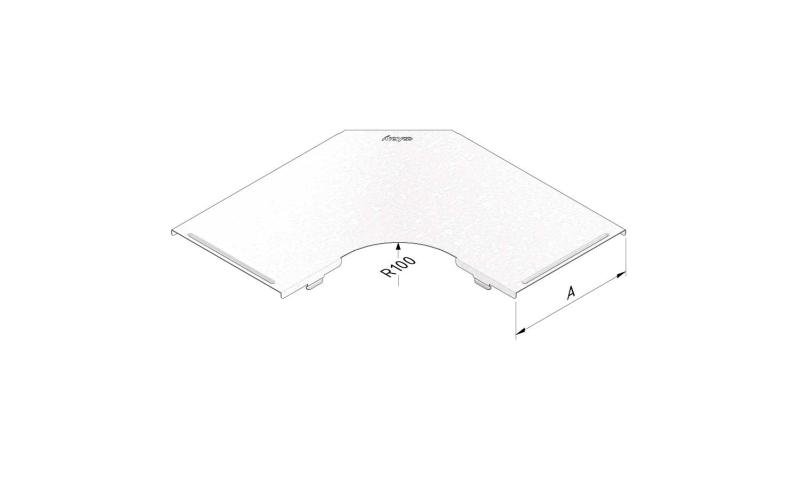
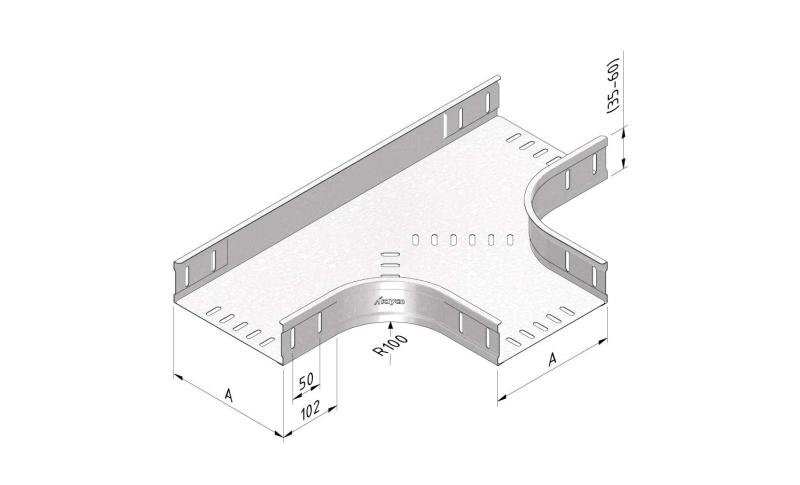
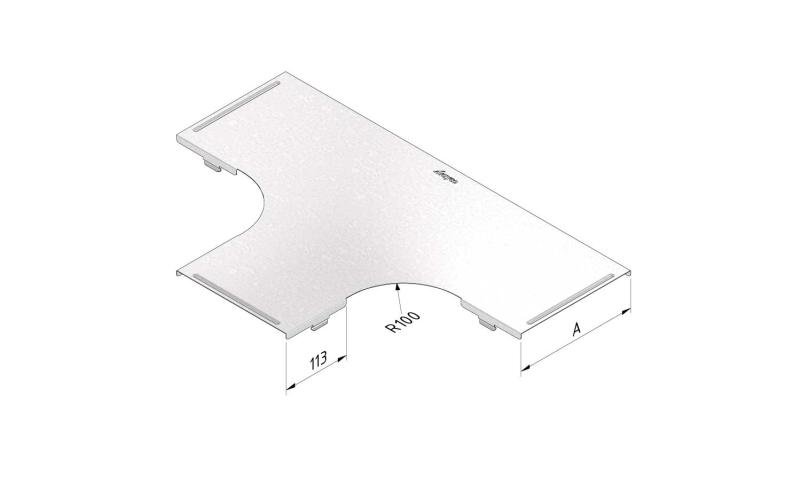
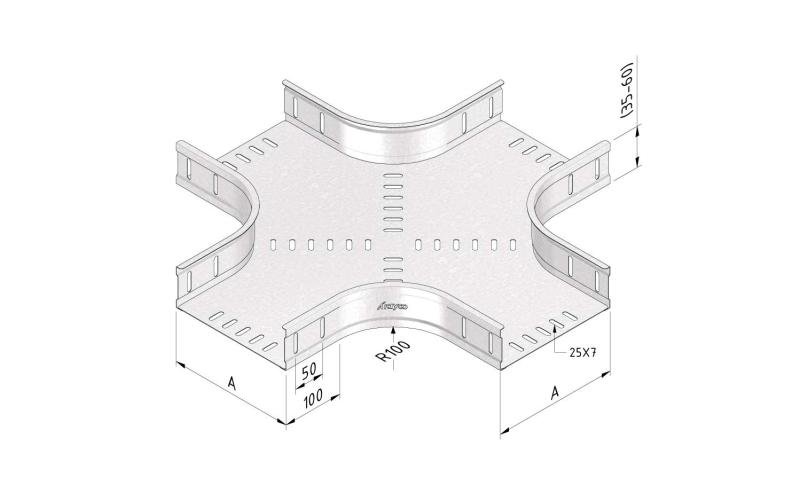
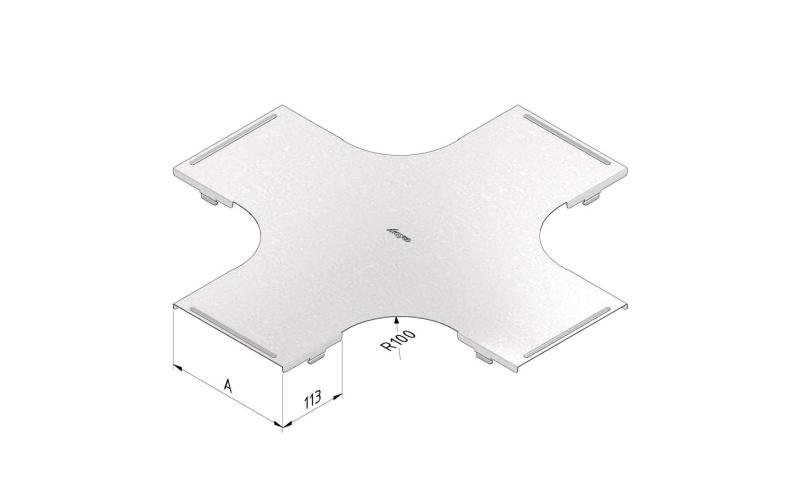
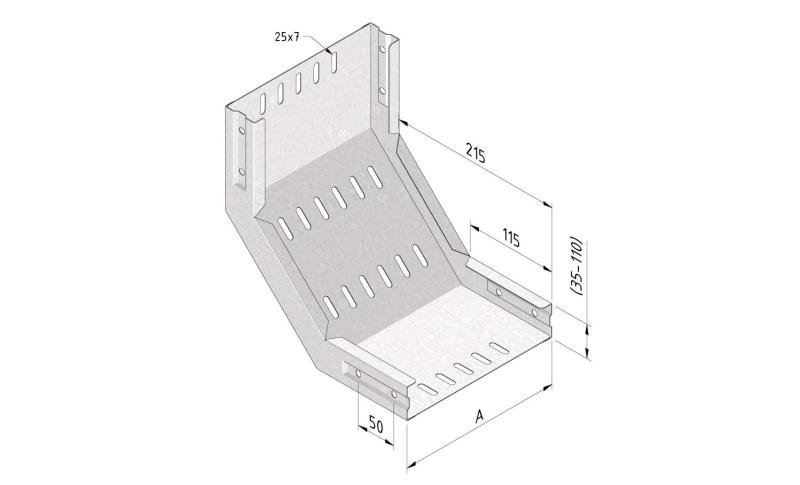
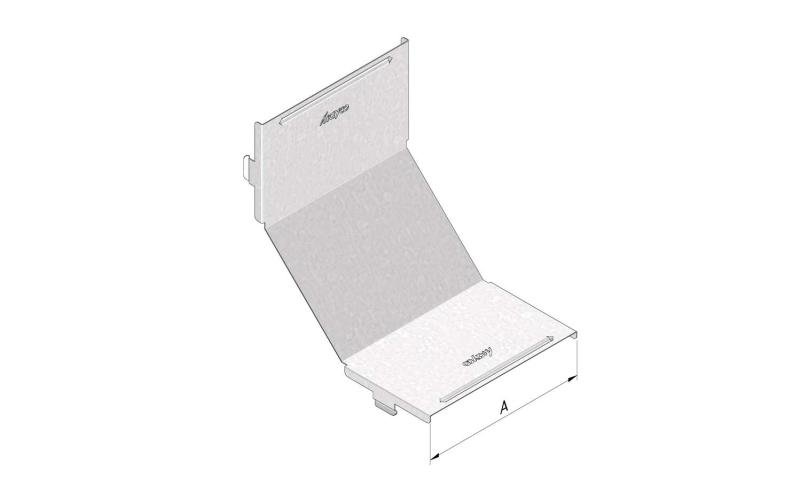
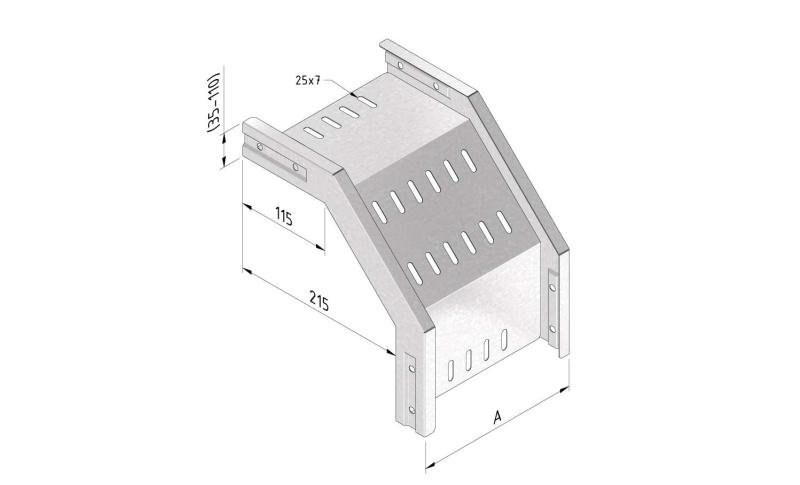
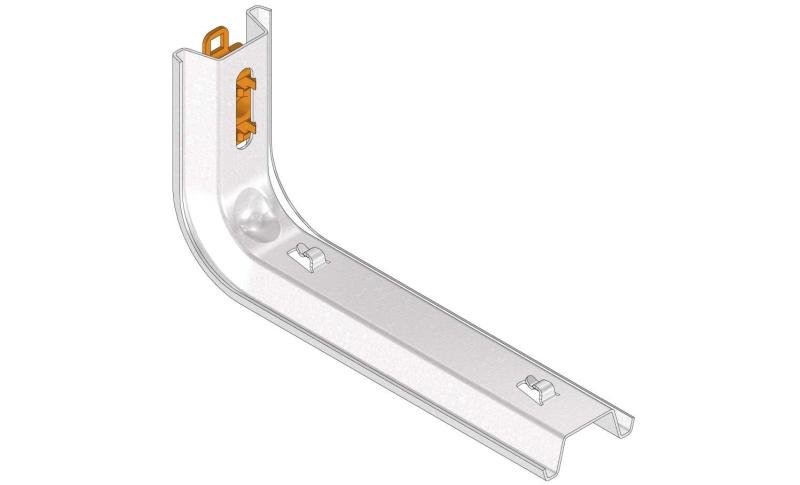
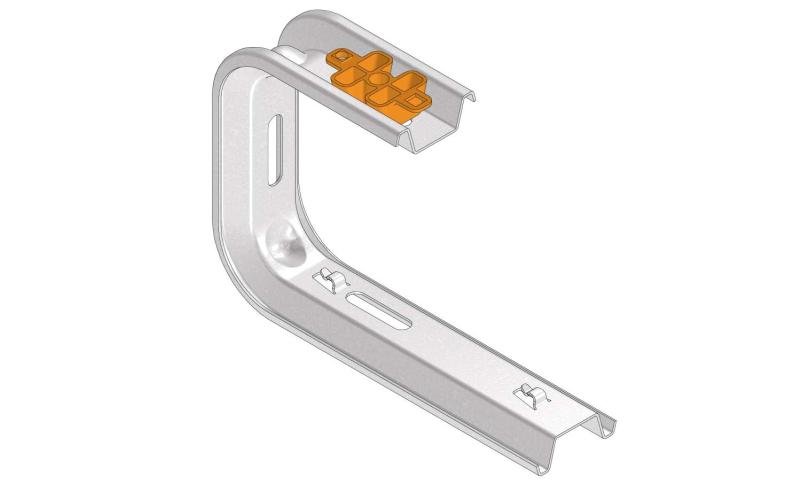
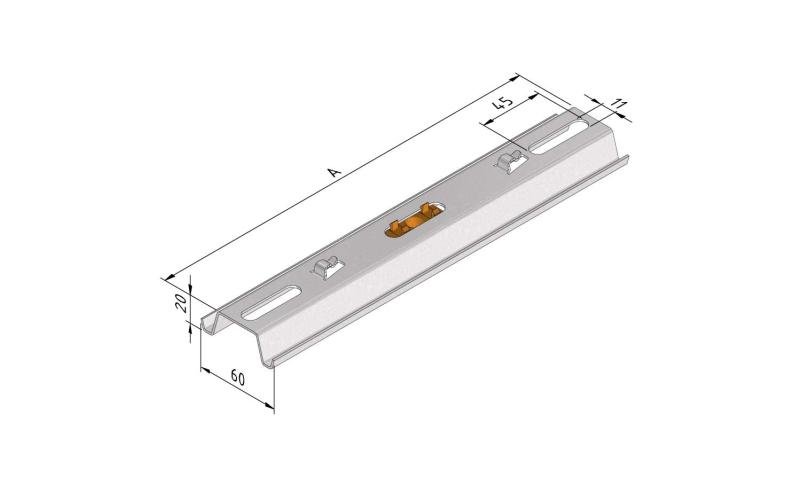
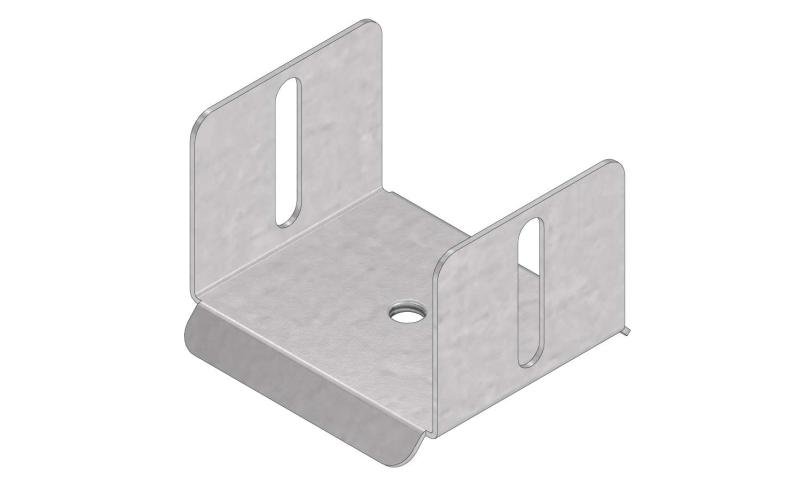
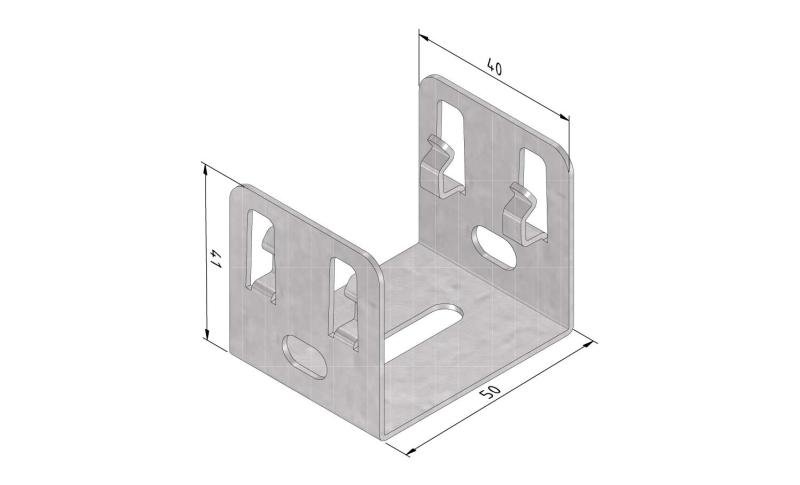
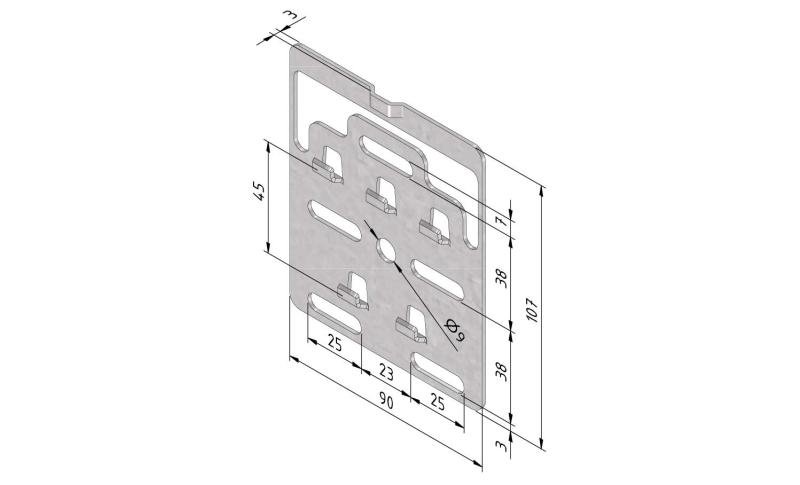
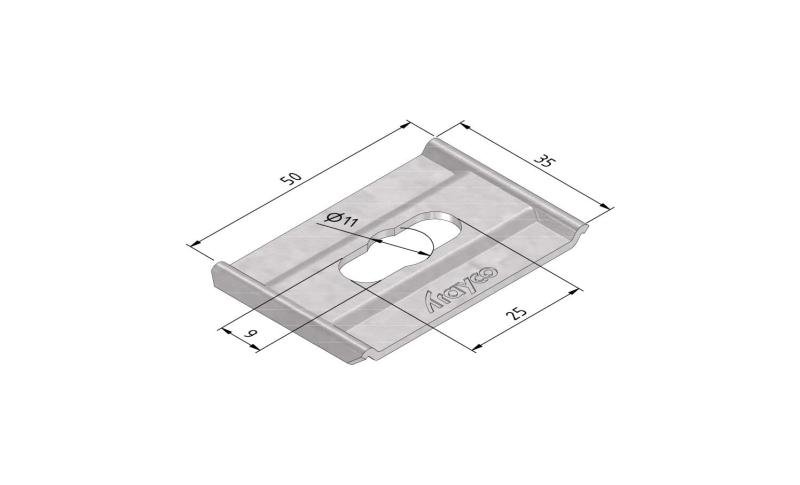
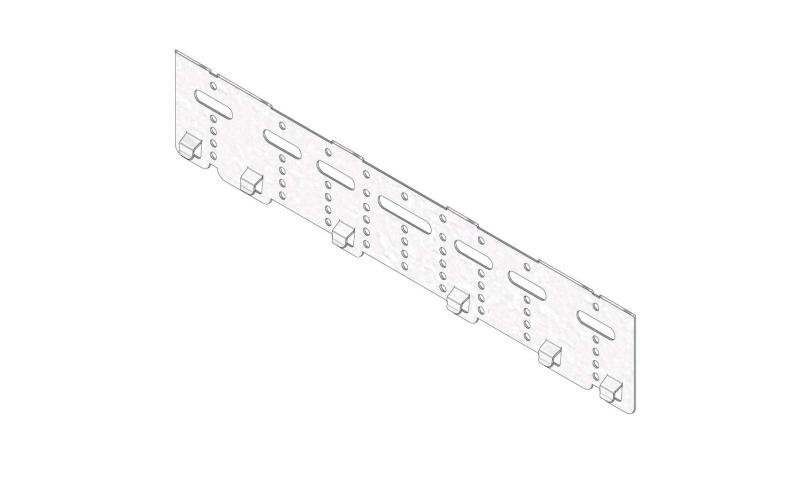
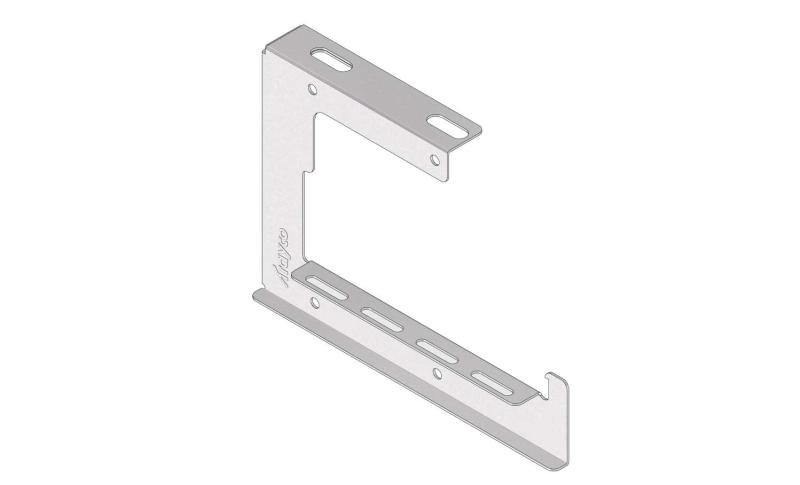
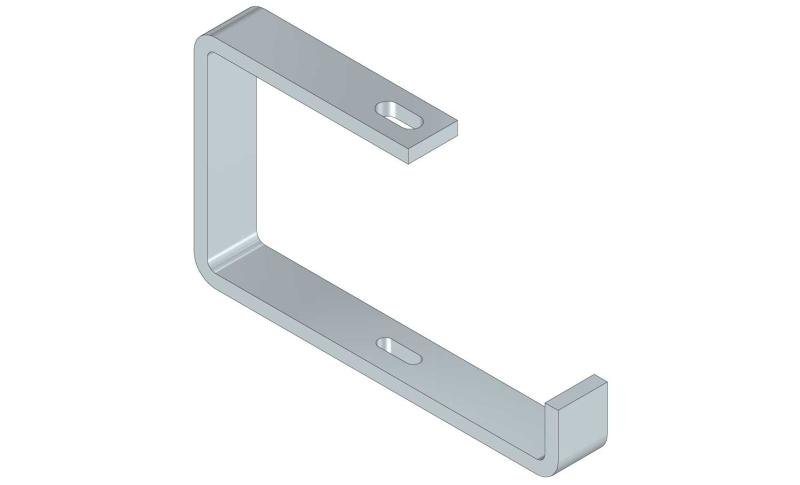
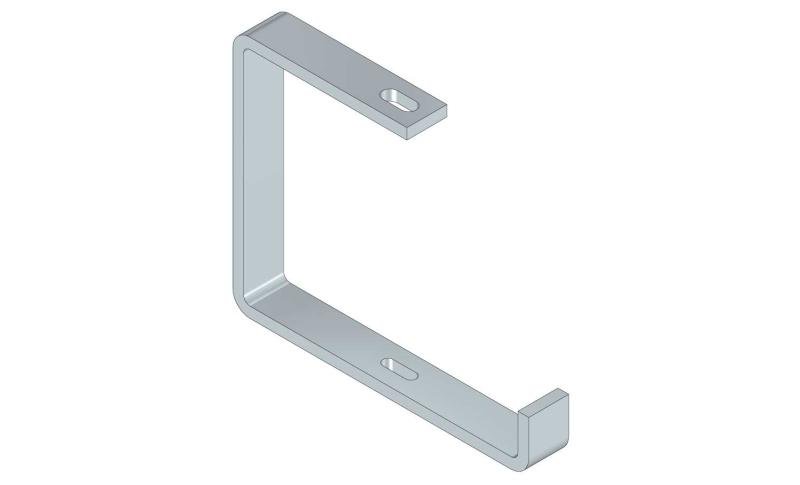
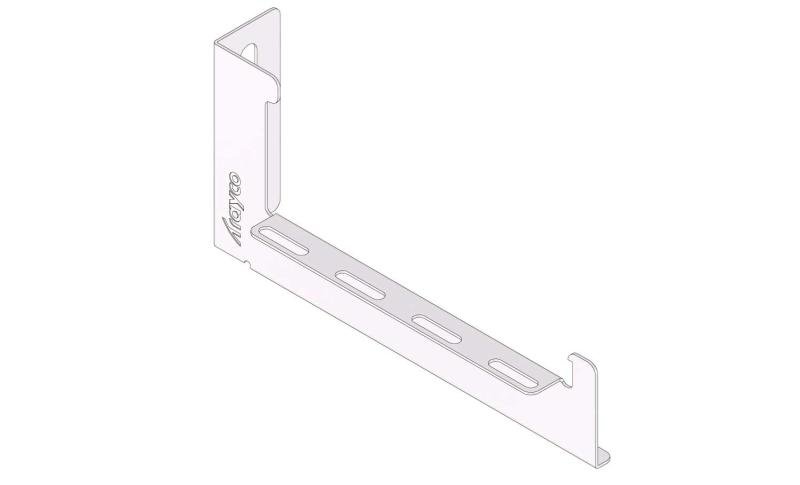
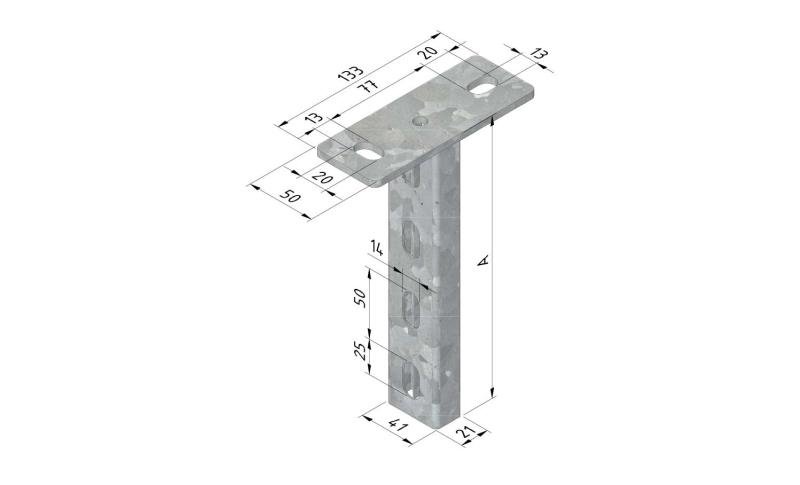
Mesh Tray
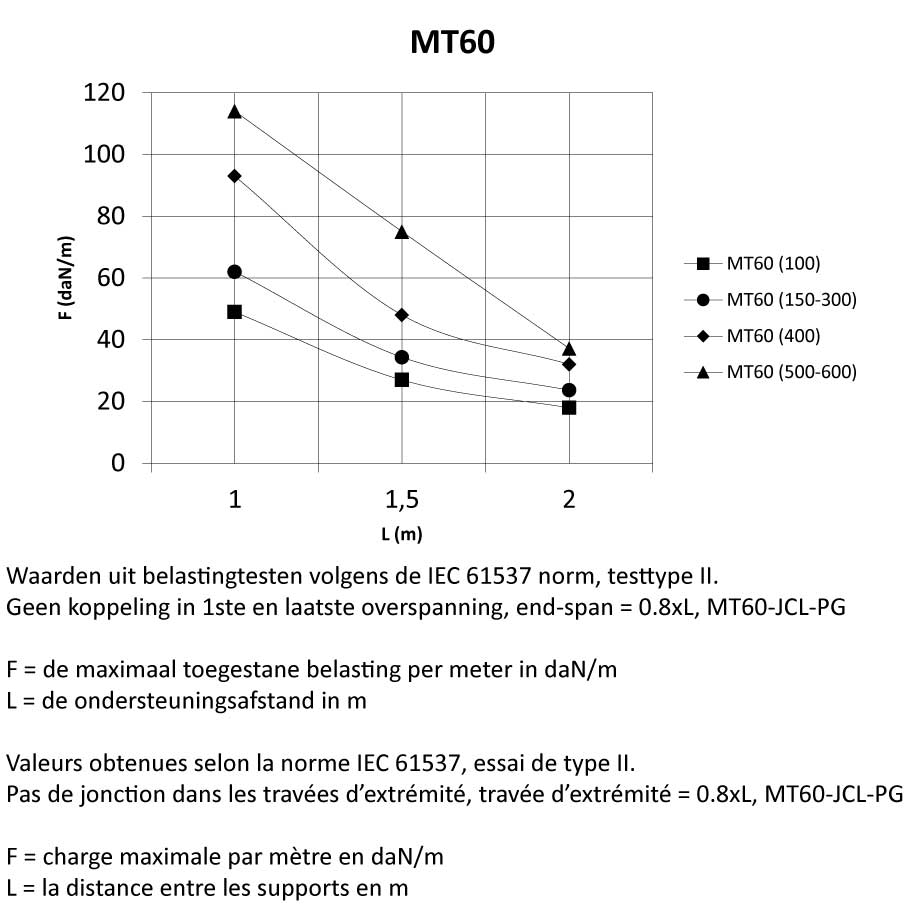
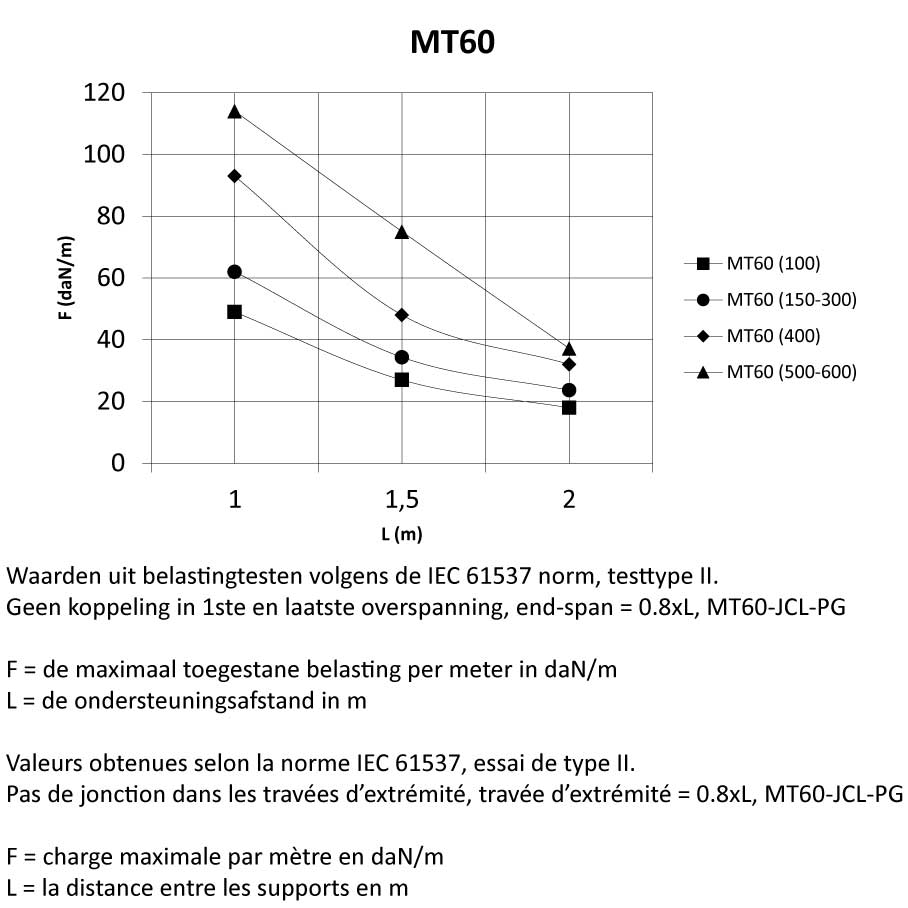
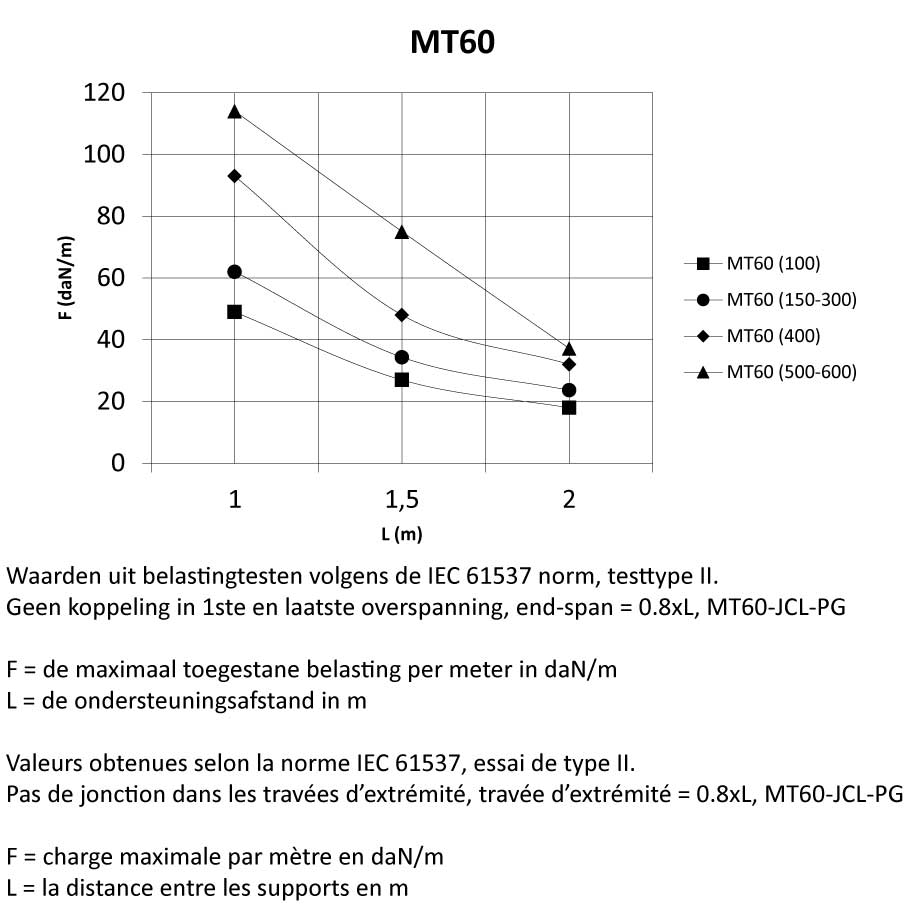
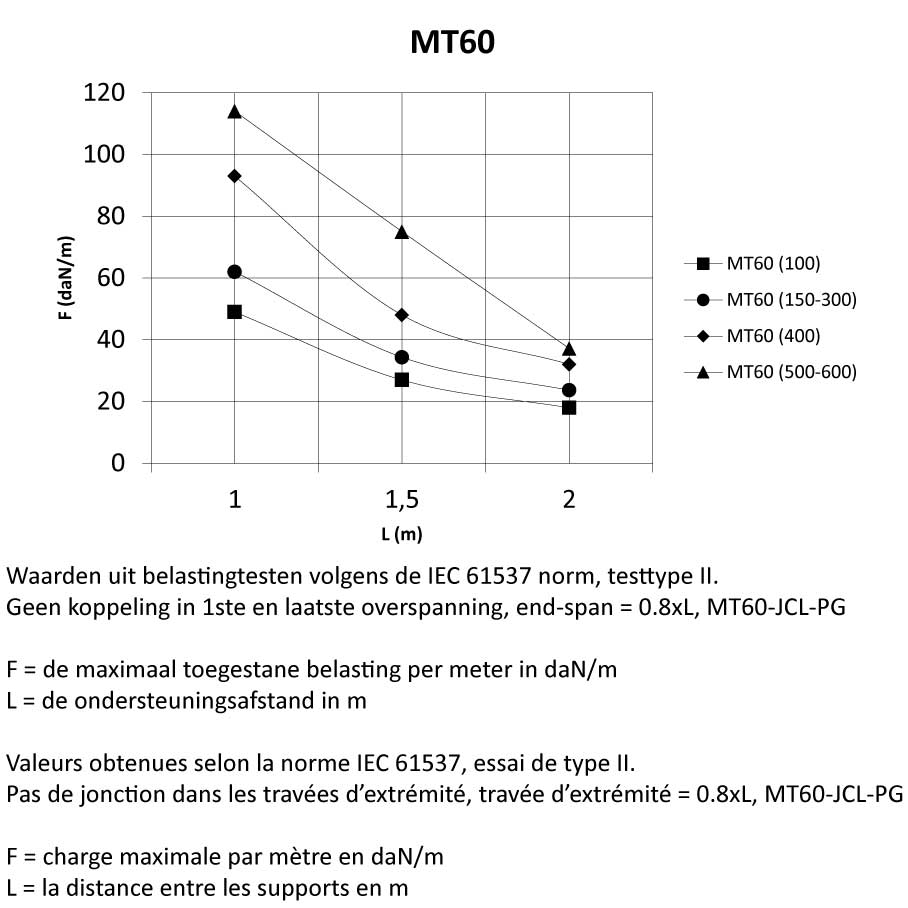
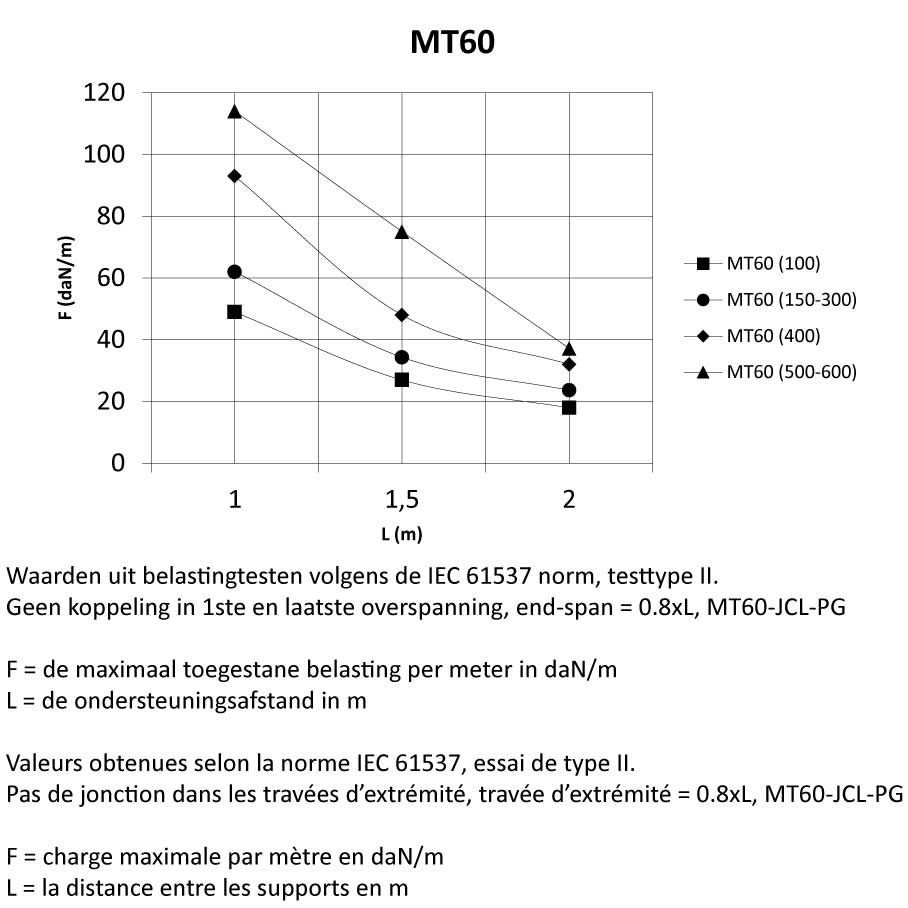
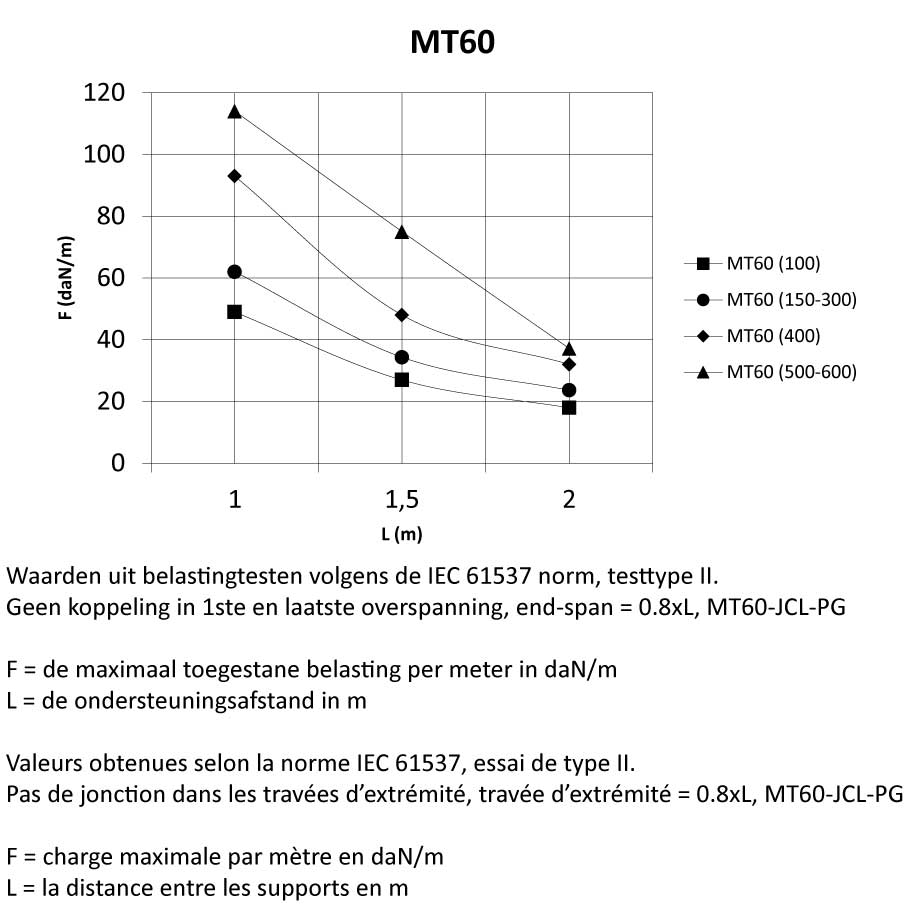
MT60

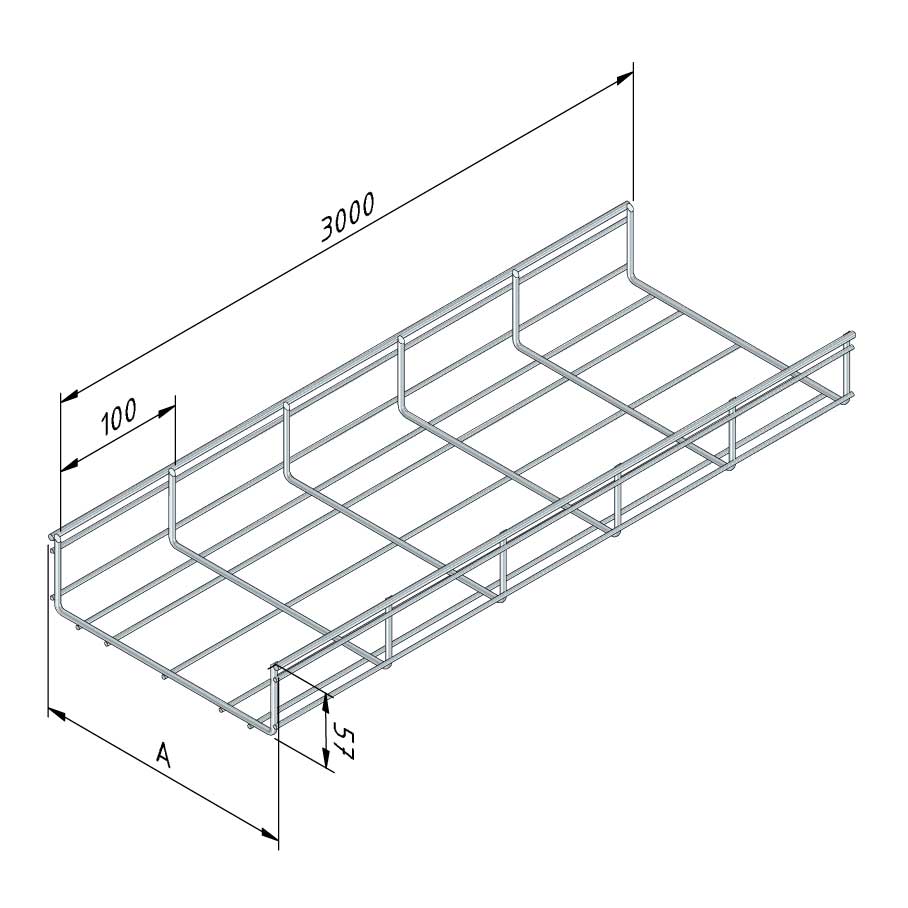
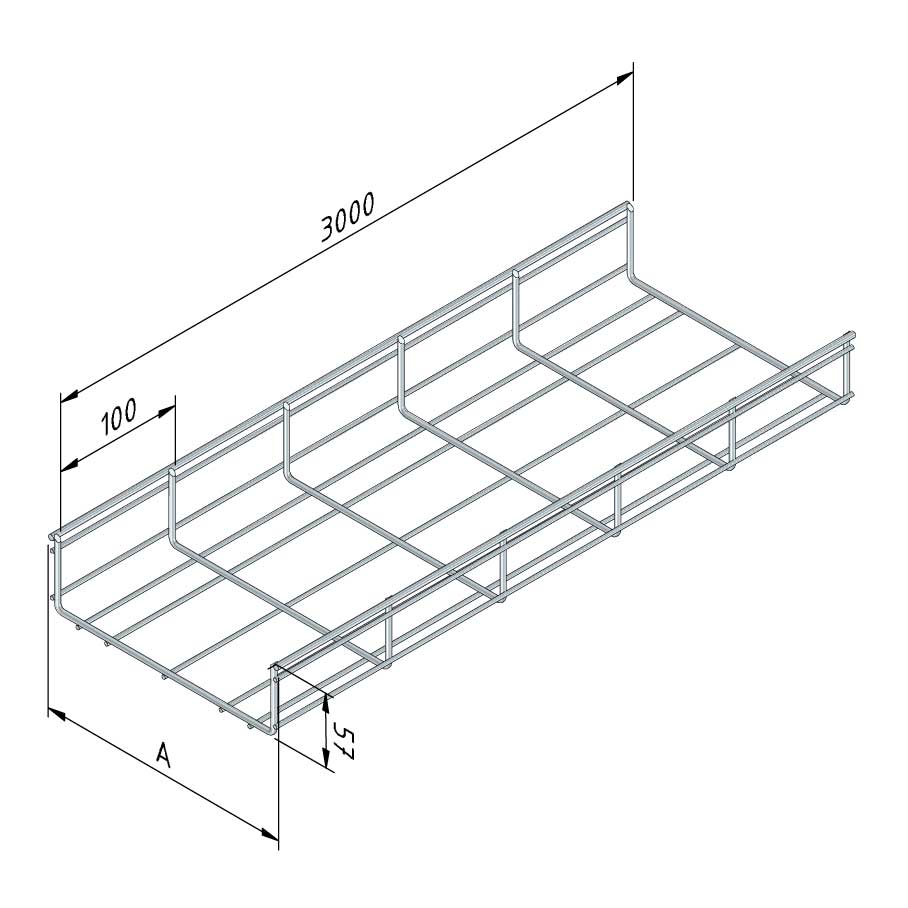
Mesh Tray
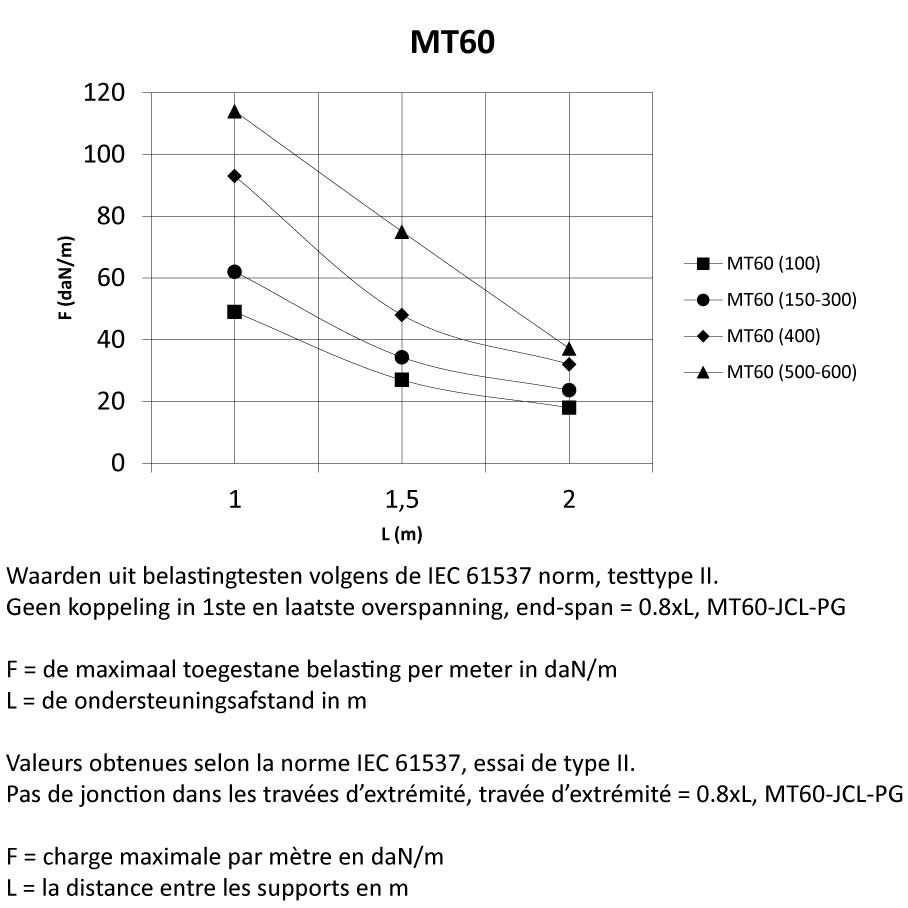
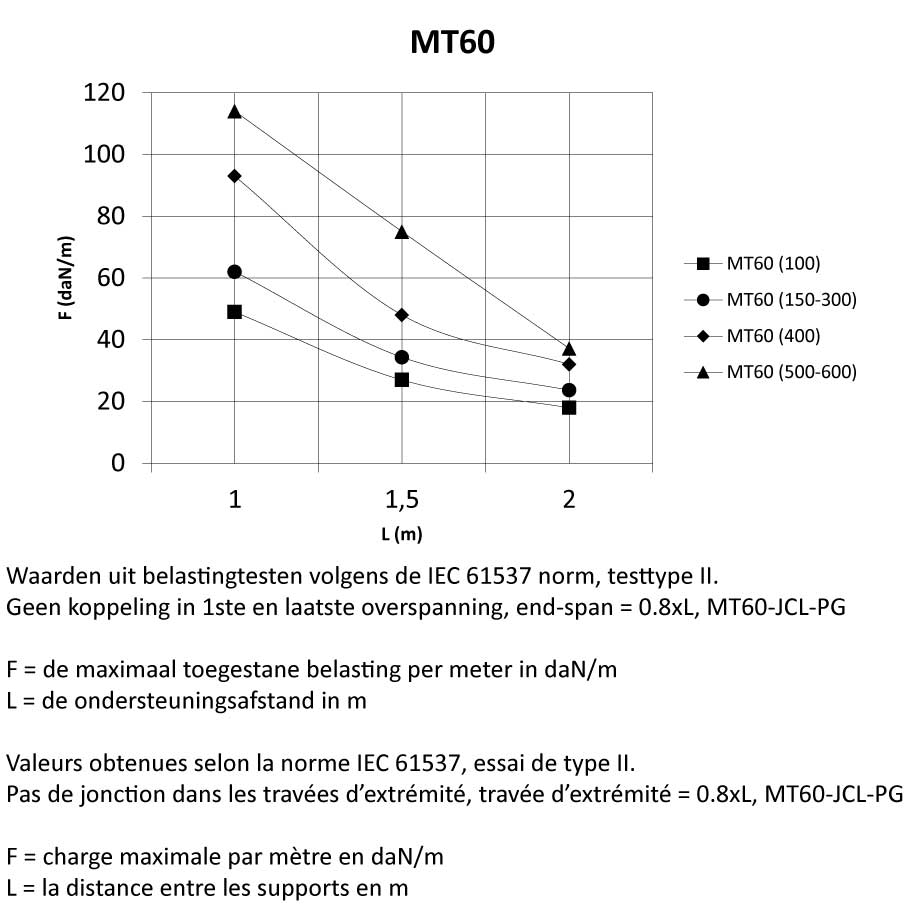
MT60

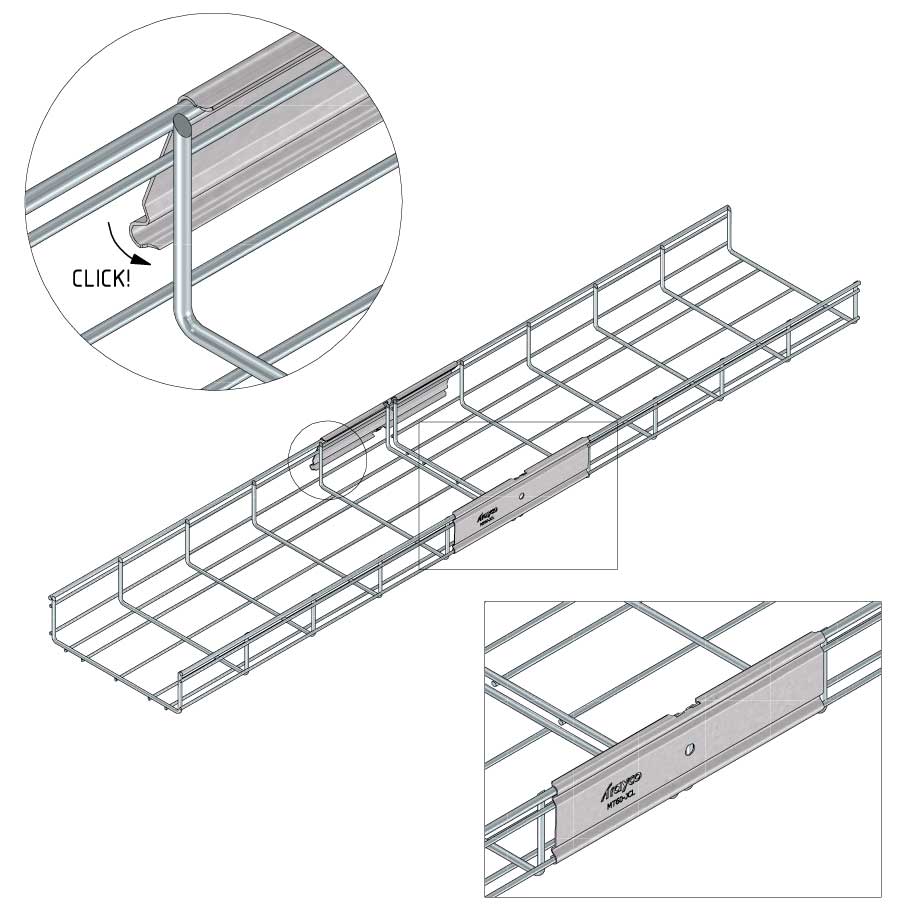
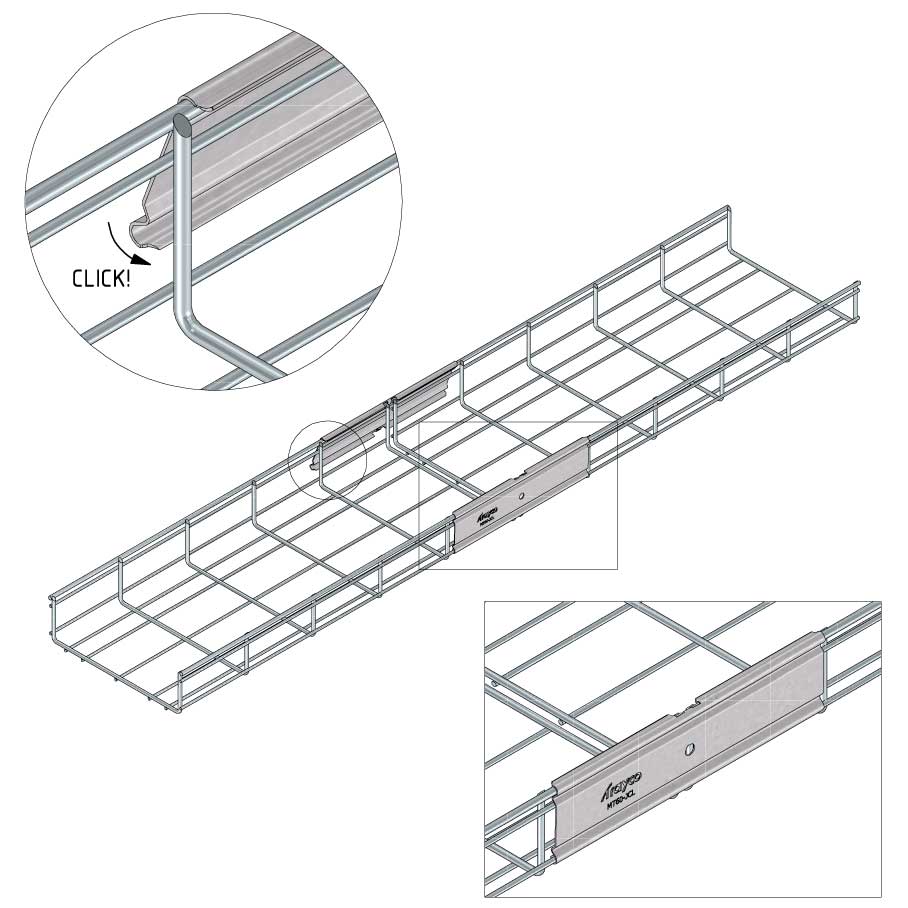
Connect with MT60-JCL
Coated finishing available on demand. RAL colour code to be confirmed on your order.
| SKU | Article code | Finishing | Dimension A | Usable surface (cm²) | Packaging | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
12264 |
MT60-050-3EG |
EG
|
50
|
16.54
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10353 |
MT60-100-3EG |
EG
|
100
|
43.67
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10358 |
MT60-150-3EG |
EG
|
150
|
70.79
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10359 |
MT60-200-3EG |
EG
|
200
|
97.92
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10360 |
MT60-300-3EG |
EG
|
300
|
152.17
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10361 |
MT60-400-3EG |
EG
|
400
|
206.42
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10347 |
MT60-500-3EG |
EG
|
500
|
260.67
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
10348 |
MT60-600-3EG |
EG
|
600
|
314.92
|
3
|
Default
|
|
|||

Additional information
Finishing
Electrolytically galvanized (EN ISO 2081) EG (electrogalvanized)
Electrolytically galvanized products are mostly used in places where limited chemical contamination is likely, for example, in off ces, industrial buildings, covered parking lots, etc. Electrogalvanizing diff ers from hot-dip galvanizing in that the zinc coating, in this case, is built up by electrolysis. With this technique, there are no thermal infl uences on the steel, so no layers of alloy will form. Also, the coating thicknesses of 6-8µm (micron) are more limited compared to hot-dip galvanizing. Prior to the galvanizing, the steel sheet goes through several pre-treatment steps so as to ensure optimal adhesion (degreasing steps, pickling, a brief acid dip, multiple rinsing,….) After the galvanizing proper, the zinc coating receives a passivating- and dichromate coat, followed by a rinsing with demi-water. The advantages of electrogalvanizing are, among other things: no thermal deformation (so ideal for assembly parts), an attractive, uniform and perfectly smooth, high-gloss f nish with good electrical conductivity, no runs in the paintwork or zinc jags. |
|||||||||||
|
|
12265 |
MT60-050-3DG |
DG
|
50
|
16.54
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10577 |
MT60-100-3DG |
DG
|
100
|
43.67
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10582 |
MT60-150-3DG |
DG
|
150
|
70.79
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10583 |
MT60-200-3DG |
DG
|
200
|
97.92
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10584 |
MT60-300-3DG |
DG
|
300
|
152.17
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10585 |
MT60-400-3DG |
DG
|
400
|
206.42
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10572 |
MT60-500-3DG |
DG
|
500
|
260.67
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
10573 |
MT60-600-3DG |
DG
|
600
|
314.92
|
3
|
|
|
|||

Additional information
Finishing
Hot-dip galvanized (EN ISO 1461) DG (dipped-galvanised):
Whenever cable support systems are exposed to the elements and/or caustic substances (such as petrochemical applications), they are given an additional treatment in the form of hot-dip galvanizing. Hot-dip galvanizing is a materials science process designed to render the steel non-corroding. If this coating is breached, the zinc will act as a sacrifcial anode, so that the iron is protected by the zinc (aka cathodic protection). During galvanization, three alloys are formed: an iron-zinc alloy, a zinc-iron alloy and also a zinc alloy. The pre-treatment of the steel is crucially important in order to achieve a good bond. The following process steps are involved: degreasing, rinsing, pickling, re-rinsing, fl uxing, drying and hot-dipping. The coating thickness depends on the steel composition, the material thickness and the time spent in the zinc bath. In the galvanizing standard NEN-EN-ISO 1461, the minimum coating thickness are prescribed (as shown in following overview), just as the zinc shrinkage per year which will depend on environmental factors (see table entitled `Corrosion classes’). In addition, the zinc coating forms an excellent substrate for other post-treatments, such as applying a powder coating and coats of paint (better known as the duplex system). An added advantage of hot-dip galvanizing is that along the edges and pointy bits, where objects are usually extra susceptible to corrosion, the zinc coating is thicker because of the behaviour of the liquid. Minimum thicknesses of the zinc coating according to ISO 1461 - Using the hot-dip method Material thickness ≥ 6 mm = min. zinc coating thickness (average) 85µm Material thickness ≥ 3 mm to < 6 mm = min. zinc coating thickness (average) 70µm Material thickness ≥ 1,5 mm to < 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 1,5 mm = min. zinc coating thickness (average) 45µm - Using the drum method Material thickness ≥ 3 mm = min. zinc coating thickness (average) 55µm Material thickness < 3 mm = min. zinc coating thickness (average) 45µm |
|||||||||||
|
|
20340 |
MT60-500-3UG |
UG
|
500
|
260.67
|
3
|
|
|
|||

Additional information
Finishing
ULTRA GALVA (UG)
This a high-performant metallic coating which offers an optimum surface protection in a wide variety of agressive and demanding environments, indoor as well as outdoor. The unique alloy of small amounts of magnesium and/or aluminium in the zinc bath provides ULTRA protection with a self-healing effect. Whilst zinc is essential for cathodic protection, magnesium prevents red rust. The passivation layer that comes on top, creates a seal that slows down the first traces of white rust. ULTRA GALVA offers a number of advantages compared to the traditional hot dip finishing. - the passivation layer offers a superior protection level. Hence, ULTRA GALVA, being cathodical, is self-healing in case of scratches, edges or perforations. Compared to hot dip, the articles remain very straight, no deflections appear nor flux or dull spots/ashes - ULTRA GALVA can conveniently be cold-processed without any risk on flakes because of the perfect adhesion of the coating to the metal - no zinc pins appear which enables one to install cables in a fast way avoiding any risk on damages to cables nor injuries of workers - thanks to the longer life span, ULTRA GALVA does not require ongoing maintenance nor post painting actions - three times less zinc is being applied compared to hot dip finishing - there is hence a lower impact on natural ressources as well as less pollution -on top, its production process generates less CO2 emission and ULTRA GALVA is 100% recyclable. ULTRA GALVA is hence a vary valuable environmentally friendly alternative for the traditional stainless steel and hot-dip finishing! |
|||||||||||
|
|
20341 |
MT60-600-3UG |
UG
|
600
|
314.92
|
3
|
|
|
|||

Additional information
Finishing
ULTRA GALVA (UG)
This a high-performant metallic coating which offers an optimum surface protection in a wide variety of agressive and demanding environments, indoor as well as outdoor. The unique alloy of small amounts of magnesium and/or aluminium in the zinc bath provides ULTRA protection with a self-healing effect. Whilst zinc is essential for cathodic protection, magnesium prevents red rust. The passivation layer that comes on top, creates a seal that slows down the first traces of white rust. ULTRA GALVA offers a number of advantages compared to the traditional hot dip finishing. - the passivation layer offers a superior protection level. Hence, ULTRA GALVA, being cathodical, is self-healing in case of scratches, edges or perforations. Compared to hot dip, the articles remain very straight, no deflections appear nor flux or dull spots/ashes - ULTRA GALVA can conveniently be cold-processed without any risk on flakes because of the perfect adhesion of the coating to the metal - no zinc pins appear which enables one to install cables in a fast way avoiding any risk on damages to cables nor injuries of workers - thanks to the longer life span, ULTRA GALVA does not require ongoing maintenance nor post painting actions - three times less zinc is being applied compared to hot dip finishing - there is hence a lower impact on natural ressources as well as less pollution -on top, its production process generates less CO2 emission and ULTRA GALVA is 100% recyclable. ULTRA GALVA is hence a vary valuable environmentally friendly alternative for the traditional stainless steel and hot-dip finishing! |
|||||||||||
Combine with (61 articles)
No results
No results were found for your current search